What is CMC Drug Development?
When it comes to drug development, the phrase ‘CMC’ stands for Chemistry, Manufacturing, and Controls. The term is commonly used in the world of pharmaceutical development, and it is an integral part of the process that ensures medications are not only effective but safe for use.
The ‘Chemistry’ part refers to the drug itself – its components and its structure. All the individual chemicals that make up a drug and the way these constituents interact with each other fall under this field. This encompasses the analytical methods used to understand the drug’s identity, purity, and strength.
‘Manufacturing’ is all about a drug’s production. It includes the methods used to create the drug consistently and reliably on a large scale. It is also where quality control measures to maintain product uniformity and consistency are implemented.
Lastly, ‘Controls’ are testing and quality assurance mechanisms. This ensures that every batch of the drug developed meets the established specifications.
CMC encompasses several foundational elements of drug development, including product and process understanding, quality control, risk management, and regulatory strategy.
Detailed Overview of the CMC Process
The CMC process plays a pivotal role in not only creating effective drugs but also ensuring a smooth trajectory toward approval by regulatory authorities.
1. Drug Formulation
The first step of the CMC process involves formulating the drug product. This is where the ‘chemistry’ part comes in; it entails identifying the ingredients that make up the drug and how they interact with each other. These include all the chemical compounds, both active and inactive.
2. Manufacturing Process
Once the formulation is established, the manufacturing process begins. This is more than just producing large quantities of the drug. It involves developing reliable and reproducible manufacturing processes, ensuring the manufacturing facility and equipment meet specified standards, and performing process validation.
3. Control Tests
As the drug moves from the lab to the manufacturing floor, another essential element – control strategies occur. These are the tests and protocols established to guarantee that each batch of medicine is consistent and of high quality. The drug evaluation includes rigorous testing to confirm that it meets certain purity and potency criteria.
The role of stability testing is also a critical component in this stage. It verifies the shelf life and storage conditions of the drug so that it remains safe and effective during this period.
4. Data Collection
The final part of CMC is the collation of all data and presentations for regulatory submissions. This includes detailed descriptions of the drug substance, its manufacturing process, control strategies, and results of stability testing.
The goal of the CMC process is to meet regulatory requirements about product quality, safety, and efficacy. It is a critical step in the complex journey of drug development, influencing everything from the initial formulation to successful regulatory approval.
Importance of CMC in the Pharmaceutical Industry
The CMC process touches every facet of drug development, making it an absolute necessity for pharmaceutical companies.
Predicts Technical and Regulatory Success
The design and execution of CMC activities can predict the technical success of drug development and, by extension, the likelihood of regulatory approval. It ensures that the drug’s manufacturing process is robust and reproducible and that the drug itself is stable and effective.
Reduces Risks
CMC also serves as a crucial risk assessment and management tool. Ensuring product quality and consistency reduces the risk of product recalls, saving pharmaceutical companies from potential financial losses and damage to their reputation.
Facilitates Innovation
The CMC process enables innovativeness in drug discovery and formulation development. By pushing pharmaceutical giants and biotech start-ups to develop more efficient production methods and drug formulations, CMC can lead to the process development of innovative medicines that better serve patients’ needs.
Ensures Compliance
With complex regulatory pathways and stringent guidelines set by regulatory agencies like the FDA, compliance is non-negotiable. A comprehensive and well-executed CMC strategy ensures that a company can meet these requirements, paving the way for a smooth and successful regulatory review and approval.
Provides a Competitive Advantage
A successful CMC strategy can also become a point of differentiation, providing a competitive advantage in the crowded pharmaceutical marketplace.
Looking at these elements, it becomes apparent that an effective CMC approach is essential in a pharmaceutical company’s path to turning drug discovery into a market-ready, safe product.
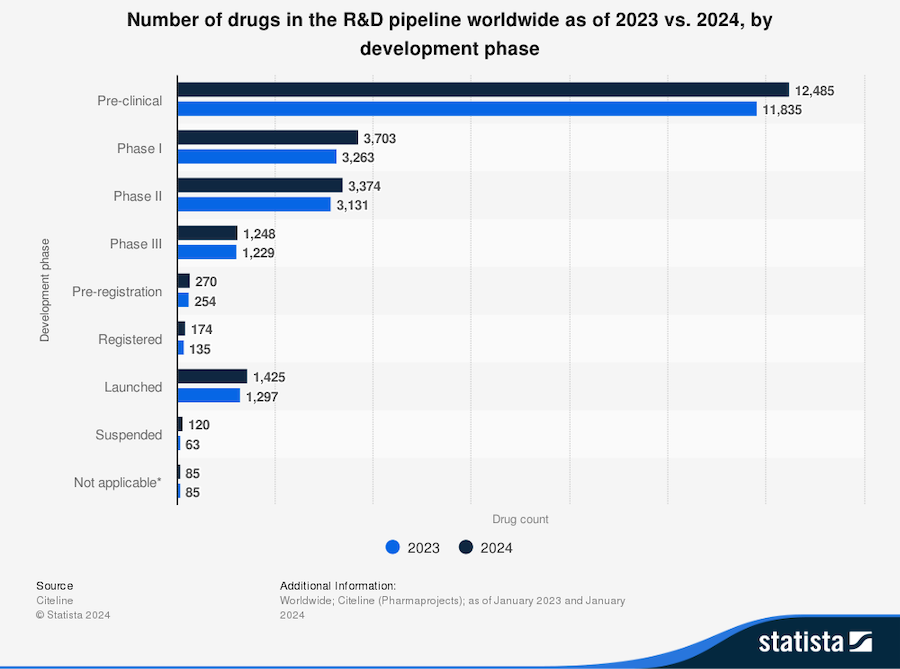
Most drugs will not successfully complete the entire process from research and development to market, due to the rigorous testing and challenges at each stage. Here is a breakdown of the drug development pipeline by phase over the past two years:
The Role of CMC in the Success of Clinical Trials
Clinical trials are the essential stages where investigational medicines are tested on human subjects for efficacy and product safety. Here’s how CMC factors into the equation.
- Ensuring Quality: At the heart of every clinical study is the investigational drug, and CMC is integral to ensuring its quality. The right manufacturing processes, controls, and analytical methods assure the safety and effectiveness of the drug being administered.
- Expediting Approvals: Good CMC practices are more likely to expedite the regulatory approval process. Detailed and well-documented CMC sections can make a big difference, advancing the review process and moving drugs to clinical development faster.
- Supporting Trial Design: CMC is not only crucial in the production of safe and efficient drugs, but it also affects the design and execution of the clinical trials themselves. For instance, efficient CMC practices can lead to the design of better dosage forms for trial subjects — ones that are easy to administer and lead to better patient compliance.
- Reducing Changes: CMC reduces the likelihood of significant changes in the production process that could affect the trial’s validity. Stability and consistency are crucial in ongoing global clinical trials, where changes in drug attributes could lead to additional studies or affect trial outcomes.
- Managing Supply Chain: Efficient CMC activities ensure a secure supply chain, essential for smooth-running clinical trials. This includes predicting and overcoming challenges in procurement, manufacturing, quality control, and distribution.
On a larger scale, CMC practices function as the backbone of successful clinical trials – ensuring the investigational medicines’ quality, speeding up regulatory approvals, and affecting the trial’s design and execution.
How Medical Packaging Inc., LLC Supports Drug Development and Clinical Trials
With a clear understanding of CMC and its importance in pharmaceutical development and clinical trials, Medical Packaging Inc., LLC (MPI) demonstrates how it meets these requirements to deliver value to its partners and customers.
As a globally recognized manufacturer of medication and pharmaceutical packaging and labeling systems, we understand the intricacies of the CMC process and its implications for medication packaging. We are committed to delivering top-quality products and providing custom-tailored medical packaging solutions that meet CMC’s stringent standards.
We offer unit dose medication packaging solutions that comply with the highest quality norms under CMC. This ensures our customers can maintain consistent product quality, a critical tendency of CMC. Our commitment to quality extends to maintaining unwavering regulatory compliance. By offering various medication packaging and labeling solutions, we help our customers meet the ever-evolving demands of regulatory agencies.
We serve a broad spectrum of healthcare settings, including hospitals, acute care facilities, specialty pharma manufacturers, and more. We provide comprehensive solutions – from oral solid and liquid medication packaging to overwrapping and labeling solutions – that cater to different needs.
By choosing MPI, pharmaceutical companies can ensure they not only stay compliant with regulatory guidelines but exceed the standards of product quality and consistency essential for successful product development and clinical trials.
Contact us today and see how we can help support your medication packaging needs.
Resources
Fontanillo, Miriam, et al. “Out of the Shadows: A Brighter Future for Pharma Technical Development.” McKinsey & Company, McKinsey & Company, 16 Sept. 2022, www.mckinsey.com/industries/life-sciences/our-insights/out-of-the-shadows-a-brighter-future-for-pharma-technical-development?utm_source=chatgpt.com.
Commissioner, Office of the. U.S. Food and Drug Administration, FDA, www.fda.gov/. Accessed 3 Dec. 2024.

Find more statistics at Statista
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients