Pharmaceutical Contract Packaging vs. In-House: Which is Right for You?

Jar filling with red tablets on a packaging line in a pharmaceutical factory – 3d illustration
Pharmaceutical repackaging offers specialized expertise and reduced capital investment but with less direct control, while in-house repackaging operations provide complete oversight and flexibility but require significant upfront investment and ongoing management. The right choice depends on your production volume, budget, and operational goals.
Healthcare organizations and pharmaceutical companies face mounting pressure to maintain high quality standards while managing costs and meeting stringent regulatory requirements. The pharmaceutical packaging process demands precise environmental controls, sophisticated tracking systems, and rigorous documentation – whether supervised internally or through contract packaging organizations. Manual packaging inefficiencies and compliance burdens continue to challenge facilities seeking optimal packaging solutions for their pharmaceutical products.
Should you outsource pharmaceutical packaging or keep it in-house? Contract packaging companies bring instant infrastructure and specialized expertise, while in-house operations deliver complete control and customization. This guide breaks down both paths to help you choose the right packaging solution for your organization.
What is In-House Medication Packaging?
In-house pharmaceutical packaging means conducting all repackaging operations with your own staff, equipment, and facilities. This approach gives hospitals, long-term care facilities, specialty pharmacy operations, and pharmaceutical companies complete control over their drug packaging processes, from primary packaging of the pharmaceutical product to secondary packaging and final distribution to patients.
Typical Setup Requirements:
In-house pharmaceutical packaging operations require infrastructure investment to address inefficient manual packaging and integrate automation into existing workflows. Organizations need specialized packaging equipment capable of handling various formats including blister pack systems, pill organizer packaging, and sterile product handling for direct contact with pharmaceutical products.
Medical Packaging Inc., LLC offers comprehensive packaging solutions for facilities choosing the in-house route, including:
- Packaging equipment for oral solids and liquids designed for seamless workflow integration
- Overwrapping systems that eliminate manual packaging inefficiencies
- Syringe labeling machines to reduce manual labeling and minimize errors
- Pak-EDGE® UD Barcode Labeling Software for enhanced compliance and operational efficiency
- Solutions scaled for both low-volume specialty pharmacy operations and high-volume pharmaceutical industry needs
Essential infrastructure components include automated packaging systems, quality control processes with serial number tracking, clean room facilities for sterile product handling, staff training programs on regulatory requirements, and quality assurance processes ensuring compliance with pharmaceutical industry standards throughout the supply chain.
Pros of In-House Packaging:
- Supply Chain Independence: In-house operations reduce dependency on external supply chain disruptions, shipping delays, and vendor availability issues. Organizations maintain direct control over their packaging timeline without relying on third-party logistics or external facility capacity, ensuring consistent production even during market challenges.
- Complete Control Over Processes and Quality: In-house repackaging operations provide direct contact with every aspect of the pharmaceutical packaging process. Hospitals, long-term care facilities, and specialty pharmacy operations maintain full oversight of quality standards, ensuring each prescription drug meets their exact specifications. This control extends to packaging materials, timing, and quality control procedures, addressing compliance burdens through direct management while maintaining patient safety throughout drug administration.
- Flexibility to Make Quick Changes: When product launches require immediate adjustments or hospital pharmacy needs change rapidly, in-house pharmaceutical packaging allows instant modifications. Staff can implement new safety features, adjust packaging formats for different drug products, or modify drug administration protocols without external coordination, eliminating delays common with manual packaging processes.
- Potential Cost Savings with High Volume: Organizations processing large amounts of units annually may achieve significant per-unit cost savings. Once initial investments are recovered, in-house repackaging operations can reduce long-term costs, especially beneficial for high-volume pharmaceutical companies and pharmaceutical manufacturers with years of experience in the pharmaceutical industry.
Cons of In-House Packaging:
- High Upfront Costs: Establishing in-house pharmaceutical packaging requires substantial capital investment. Equipment, facility modifications, staff training, and regulatory compliance infrastructure represent significant financial commitments before processing the first pharmaceutical product. This challenge particularly affects independent pharmacies and specialty pharmacy operations with limited capital resources.
- Regulatory Burden and Compliance Challenges: Maintaining compliance with FDA regulations and pharmaceutical industry standards requires dedicated expertise. Organizations must stay current with changing regulatory requirements, conduct regular audits, and maintain extensive documentation for every drug product processed. MPI’s FDA-accepted Type III Drug Master File can support speed to market and compliance efforts, but internal expertise remains essential for pharmaceutical repackaging operations.
- Time Consuming for Healthcare Staff: In-house repackaging operations demand significant staff resources. Hospital pharmacy teams, long-term care facilities, and specialty pharmacy operations must dedicate personnel to packaging tasks rather than focusing exclusively on patient care and drug administration, potentially impacting operational efficiency and reducing time available for direct patient care.

What are Contract Repackaging Companies?
Contract repackaging companies are specialized pharmaceutical packaging companies that manage repackaging operations for healthcare facilities and pharmaceutical manufacturers. These repackaging services allow hospitals, specialty pharmacy operations, pharmaceutical companies, and contract manufacturing organizations to outsource their packaging needs to experts with years of experience addressing inefficient manual packaging and compliance burdens in the pharmaceutical industry.
How It Works: Healthcare organizations send their pharmaceutical products to the contract packaging facility, where experienced teams oversee all aspects of pharmaceutical packaging using state-of-the-art equipment and smart packaging technologies. The facility processes medications according to strict quality standards and regulatory requirements, then returns the finished output packages ready for distribution to patients, eliminating manual packaging inefficiencies while maintaining patient safety.
Pros of Contract Repackaging:
- Expertise & Compliance Already in Place: Contract packaging companies bring years of experience and specialized knowledge to every pharmaceutical product, addressing the compliance burdens that challenge healthcare facilities. These pharmaceutical packaging companies maintain dedicated compliance teams, ensuring all regulatory requirements are met without clients needing to develop internal expertise. Their facilities are designed specifically for pharmaceutical repackaging, with proper environmental controls and quality systems that eliminate labeling errors while maintaining high quality standards.
- Scalability for Any Volume: Whether processing small batches for specialty pharmacy operations or large volumes for pharmaceutical manufacturers, contract repackaging services can scale to meet demand. This flexibility accommodates seasonal variations, product launches, and changing market conditions without requiring clients to invest in additional capacity, solving automation challenges for both low- and high-volume needs across the pharmaceutical industry.
- Frees Up Staff Time for Patient Care: By outsourcing repackaging operations, hospital pharmacy staff, long-term care facilities, and specialty pharmacy teams can focus on direct patient care and drug administration rather than addressing inefficient manual packaging. This allows healthcare professionals to dedicate more time to patients while ensuring pharmaceutical packaging is managed by compliance specialists with expertise in prescription drug handling.
- Enhanced Operational Efficiency: Contract packaging eliminates the workflow integration challenges that plague many healthcare facilities. Professional pharmaceutical packaging companies have optimized processes that reduce labeling errors, improve turnaround times, and ensure consistent quality standards across all pharmaceutical products while maintaining patient safety throughout the supply chain.
Cons of Contract Repackaging:
- Less Control Over Turnaround Times and Customization: While contract packaging companies work to meet client timelines, customers have less direct control over scheduling and specific customization requests compared to in-house operations. Changes may require coordination and additional lead time, potentially impacting urgent needs at hospitals and specialty pharmacy operations.
- Ongoing Costs: Contract repackaging involves predictable service fees, shipping costs, and per-unit charges that continue throughout the partnership. While these eliminate upfront capital investment, they represent ongoing operational expenses that may impact budgets for healthcare facilities, independent pharmacies, and specialty pharmacy operations.
- Dependency on Vendor Reliability: Success in pharmaceutical packaging relies on performance, quality standards, and meeting delivery commitments. Healthcare facilities must carefully evaluate potential partners to ensure reliability and compliance capabilities that meet their operational requirements for pharmaceutical products and patient safety. Organizations also become vulnerable to supply chain disruptions, including shipping delays, capacity constraints, and external vendor issues beyond their direct control.
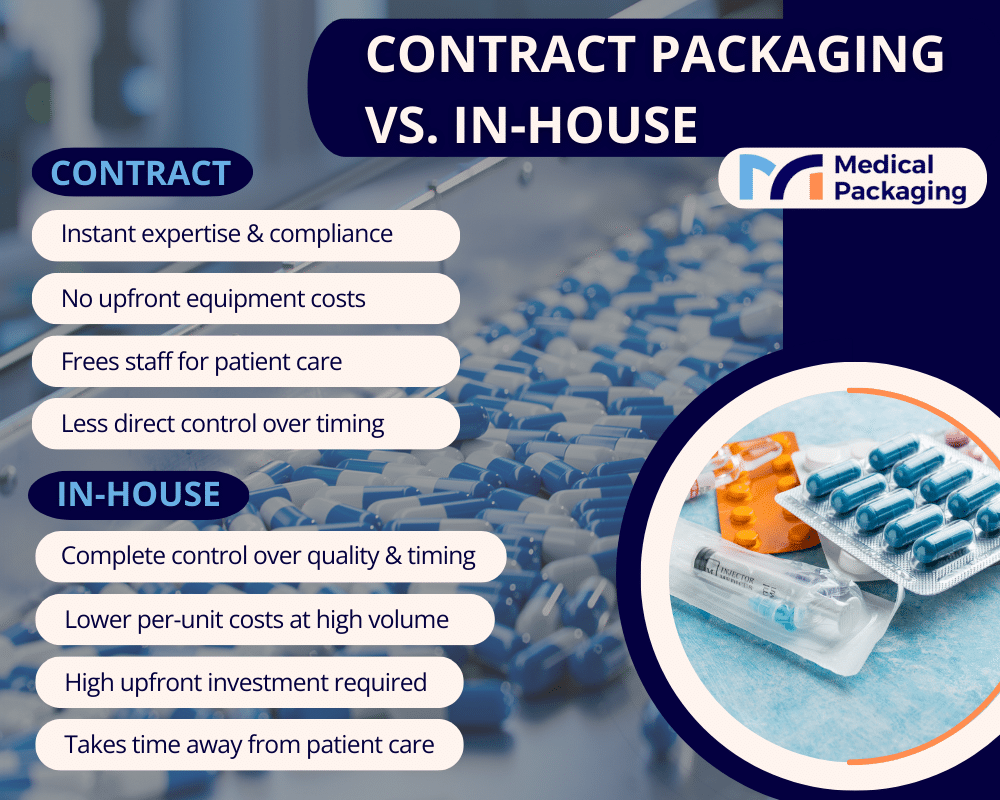
Making the Financial Decision: In-House vs. Contract Packaging
Understanding the financial implications helps healthcare organizations and pharmaceutical companies make informed decisions about their packaging strategy, particularly when addressing inefficient manual packaging and compliance burdens while maintaining high quality standards for pharmaceutical products.
In-House Packaging Costs:
- High startup costs: Significant capital investment for packaging equipment, facility modifications, staff training, and compliance infrastructure
- Lower long-term per-unit costs: Once initial investments are recovered, high-volume repackaging operations may achieve favorable per-unit economics
- Best for: Pharmaceutical manufacturers, large pharmaceutical companies, and hospital systems with consistent medication packaging needs and stable drug product lines
Contract Repackaging Costs:
- No startup costs: Immediate access to pharmaceutical packaging capabilities without capital investment, eliminating barriers for specialty pharmacy operations and independent pharmacies
- Predictable ongoing fees: Clear per-unit pricing that includes all operational costs, materials, and compliance management for pharmaceutical products
- Best for: Variable volumes, seasonal products, specialty pharmacy operations, long-term care facilities, and organizations seeking to preserve capital while eliminating manual packaging inefficiencies
The Strategic Decision: Many successful healthcare organizations use hybrid approaches – maintaining in-house capabilities for core drug products while partnering with contract packaging organizations for overflow capacity, product launches, or specialized requirements like sterile products and complex pharmaceutical packaging formats that require direct contact with sensitive pharmaceutical products.
Medical Packaging Inc., LLC (MPI) serves as a leading global provider of unit dose pharmaceutical packaging and labeling systems. With a customer base spanning hospitals, long-term care pharmacies, pediatric facilities, and pharmaceutical manufacturers, MPI’s comprehensive product line includes specialized packaging systems for oral solids and liquid medications, syringe labeling, overwrapping, and the proprietary Pak-EDGE® UD Barcode Labeling Software – all designed to enhance patient safety and ensure regulatory compliance.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients