Track and Trace in the Pharma Industry

Pharmaceutical track and trace systems have become the gold standard for supply chain security, protecting medications from manufacturing through patient dispensing. With the World Health Organization estimating that 10.5% of medicines worldwide are substandard or falsified, these sophisticated technologies create an unbroken chain of verification that effectively combats the growing threat of counterfeit medications while ensuring regulatory compliance.
The scale of this problem is evident in recent enforcement actions. Europol’s Operation SHIELD IV seized counterfeit and misused medicines worth over €64 million between April and October 2023, resulting in 1,284 individuals being charged across 30 countries. For pharmacies, hospitals, and healthcare facilities, implementing robust track and trace solutions not only protects patients but also streamlines operations through enhanced inventory visibility and automated authentication.
This guide explores how track and trace works, the challenges involved in implementation, and why Medical Packaging Inc., LLC (MPI)’s Pak-EDGE® solution offers the ideal combination of compliance, security, and operational efficiency for healthcare providers.
What is Track and Trace?
In today’s pharmaceutical landscape, ensuring medication integrity from production to end user is critical. Track and trace technology has emerged as the industry’s defense against counterfeit drugs, supply chain disruptions, and regulatory non-compliance.
These systems provide unprecedented visibility into pharmaceutical supply chains that have grown increasingly global. From manufacturing to dispensing, each handoff point represents a potential vulnerability. Track and trace illuminates these transitions, creating an unbroken chain of accountability that reduces the risk of counterfeit products entering legitimate channels.
Beyond compliance, track and trace benefits the entire pharmaceutical industry ecosystem. Manufacturers gain enhanced inventory control and brand protection. Distributors benefit from streamlined processes and reduced liability. Healthcare providers can verify medication authenticity instantly, while patients receive an additional layer of protection against harmful counterfeit drugs.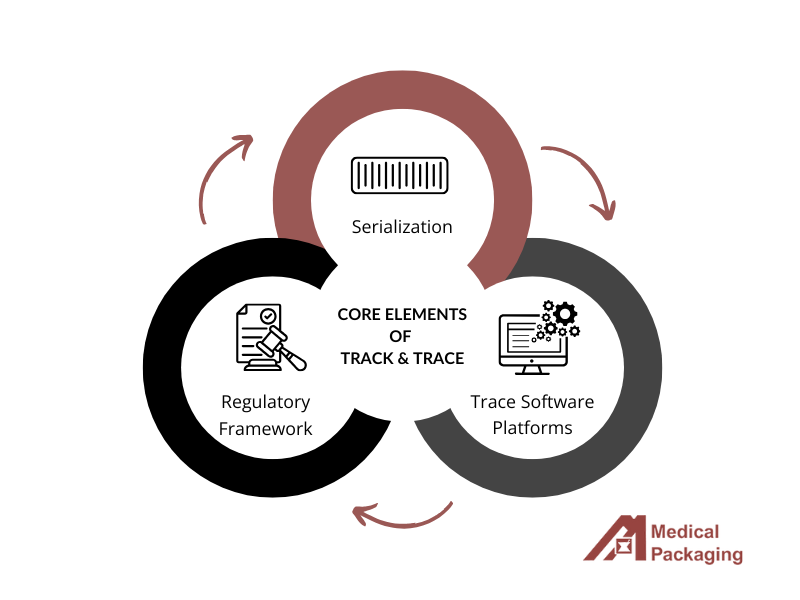
How Track and Trace Works in the Pharmaceutical Supply Chain
Track and trace systems rely on three key components working together. First, pharmaceutical serialization assigns unique identifiers to each medication package through product codes, unique serial numbers, and batch information. These are applied using specialized barcode labeling software integrated with high-precision packaging equipment.
Second, trace software platforms track products as they move through the supply chain. When items change hands, scanning creates digital transaction records that update in real-time. This allows pharmacists to verify authenticity instantly and receive automatic recall notifications.
Third, a complex regulatory framework drives implementation globally. The European Union’s Falsified Medicines Directive, and the U.S. Drug Supply Chain Security Act, each have unique requirements. Pharmaceutical companies must navigate these varying regulatory authorities while maintaining operational efficiency across markets, making flexible, compliant systems essential for global distribution.
How Pharmacies, Hospitals, and Healthcare Facilities Can Use Track and Trace
For pharmacies, track and traceability systems transform medication management with immediate benefits. Staff can instantly verify product authenticity upon delivery, confirming medications are not counterfeit, expired, or recalled. The system enables precise inventory management with automated expiration tracking and creates an auditable trail that simplifies regulatory compliance.
In hospital settings, track and trace provides complete visibility of medication movement throughout the facility. Central pharmacies can optimize inventory across satellite locations and patient care areas, reducing both carrying costs and emergency orders. When integrated with electronic health records, scanning medications before administration verifies the right patient receives the right medication, reducing errors.
Track and trace implementation shows measurable improvements in healthcare settings. According to industry observations, pharmacies experience significant reductions in recall processing time, while hospitals report faster medication retrieval and fewer stocking errors. Healthcare facilities can better document returned medications for proper reimbursement, and long-term care facilities typically see reduced medication waste through improved inventory visibility and management.
Challenges of Pharmaceutical Track and Tracing
Implementing track and trace systems presents significant challenges for pharmaceutical companies. The fragmented regulatory landscape requires navigating different standards across regions—from the EU’s FMD to U.S. DSCSA regulations. Global manufacturers must create adaptable systems that accommodate multiple standards while maintaining constant compliance with evolving requirements.
Meanwhile, sophisticated counterfeiters continuously develop methods to circumvent protections, from tampering with packaging to creating convincing duplicate barcodes. This requires ongoing security enhancements throughout the supply chain.
Technical integration with legacy systems presents another obstacle. Older production equipment often lacks the connectivity required for serialization, making retrofitting existing packaging lines costly and complex.
The massive data volumes generated require robust master database architectures and governance frameworks. Beyond the substantial initial equipment and software investments, organizations face ongoing expenses for maintenance, upgrades, and staff training. While large manufacturers can absorb these costs, smaller companies often struggle with the investment required, potentially limiting market participation.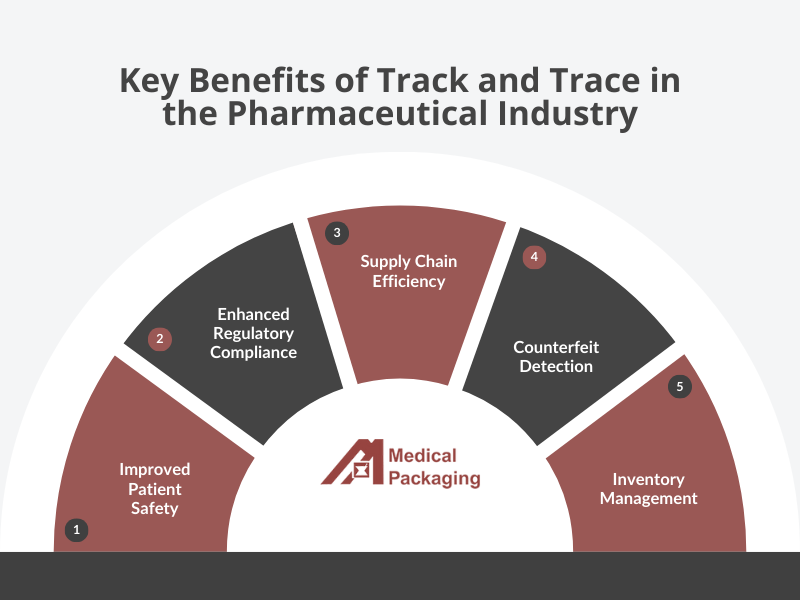
Safeguarding Medicine: Why Track and Trace Matters
Track and trace systems create powerful protection against counterfeit medicine by establishing an unbroken verification chain. Each serialized package carries a digital pedigree that can be authenticated anywhere in the supply chain, immediately flagging suspicious pharmaceutical products before they reach patients.
For pharmaceutical manufacturers, this technology provides essential brand protection. Counterfeit products not only represent lost revenue but can severely damage reputations when patients experience adverse effects from fake medications bearing trusted names.
These systems also establish the accountability framework essential for regulatory compliance. The ability to document every product’s journey provides comprehensive records required by authorities worldwide, streamlining inspections while enabling targeted, efficient recalls when necessary.
For patients—the ultimate beneficiaries—track and trace means improved safety and confidence. Every verification scan provides an additional layer of protection, creating peace of mind for both patients and healthcare providers that medications have followed secure, documented pathways from manufacturer to pharmacy.
Elevate Your Traceability: Why MPI’s Pak-EDGE Is the Smart Choice
Our Pak-EDGE® UD Barcode Labeling Software is a comprehensive track and trace solution designed specifically for pharmacies, hospitals, and healthcare facilities. This software solution provides a single-user interface for all MPI packaging systems, creating a cohesive ecosystem for pharmaceutical inventory management.
Pak-EDGE offers healthcare providers robust traceability through advanced barcoding functionality, supporting 1D, 2D, and GS1 formats essential for global compliance. Its centralized database allows facilities to maintain consistent data across departments, while customizable label design ensures every package meets facility requirements while maintaining regulatory standards. The system’s batch approval components enable proper oversight, and enhanced security features prevent unauthorized access.
By choosing Pak-EDGE, healthcare facilities gain operational efficiency through comprehensive drug libraries, detailed reporting tools, and seamless integration with MPI’s packaging equipment. This complete ecosystem not only reduces the risk of counterfeit medications but also positions facilities at the forefront of medication safety and regulatory compliance.
Ready to transform your medication management? Contact MPI today to discover how Pak-EDGE can enhance traceability, ensure compliance, and improve patient safety.
Content
- What is Track and Trace?
- How Track and Trace Works in the Pharmaceutical Supply Chain
- How Pharmacies, Hospitals, and Healthcare Facilities Can Use Track and Trace
- Challenges of Pharmaceutical Track and Tracing
- Safeguarding Medicine: Why Track and Trace Matters
- Elevate Your Traceability: Why MPI’s Pak-EDGE Is the Smart Choice
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients