Medical Equipment Planning
As technological advancements continue to become more complex and the healthcare industry races to keep up, proper planning of medical equipment is becoming increasingly vital. Imagine building a state-of-the-art healthcare facility, complete with every modern convenience, but finding that you have not carefully planned for the medical equipment needed. This could lead to operational inefficiencies and compromise patient safety, a risk that no healthcare facility can afford to take.
For this reason, the role of a medical equipment planner is becoming increasingly vital in the healthcare industry. Through meticulous planning, these professionals ensure that not only are the needs of today met but that future requirements are also accounted for.
Medical equipment planning could lead to significant improvements in patient care, safety, and overall operational efficiency.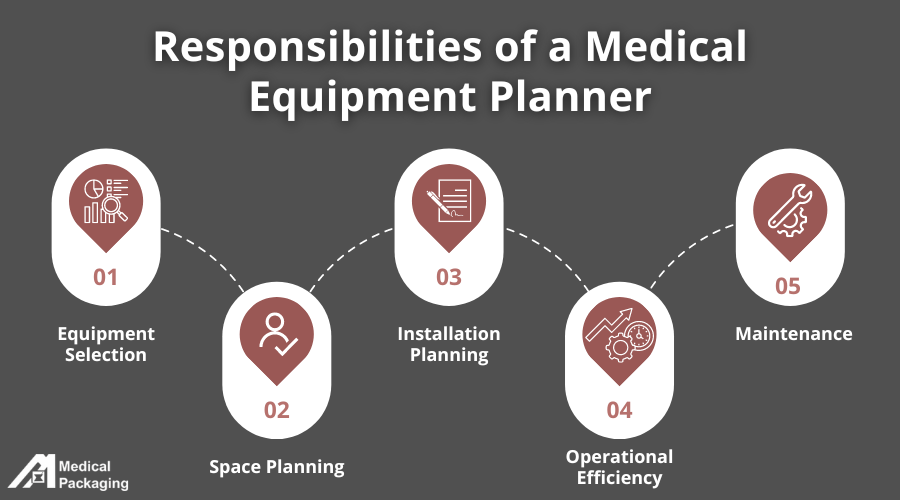
Responsibilities of a Medical Equipment Planning Team
The medical equipment planning process is a comprehensive and strategic process that determines the distinct medical equipment needs for any healthcare facility, be it an existing establishment, a renovation project, or larger-scale healthcare construction projects. This essential process covers a broad spectrum of tasks that combine to bring everything together smoothly.
A vital player in this process is the medical equipment planner whose role is multifaceted, encompassing:
1. Equipment Selection
A medical equipment planner often starts with an understanding of the healthcare service’s needs that the medical facility aims to provide. This understanding helps to guide the selection of necessary medical equipment, packaging materials, and machines.
2. Space Planning
The planner works closely with architects and builders to ensure that the spatial allocation for each piece of equipment is appropriate and conducive for both the staff and patients.
3. Installation Planning
This includes considering factors like power requirements, climate controls, and necessary peripherals, and ensuring the equipment’s integration within the healthcare facility is seamless.
4. Operational Efficiency
The planner should devise efficient workflows around the designated medical equipment to enhance patient outcomes, reduce errors, and maximize patient comfort.
5. Maintenance Plans
A crucial part of the equipment planner’s job is to plan for the regular maintenance of the equipment. This ensures the appliances stay functional and last longer.
The role and expertise of an effective medical equipment planner extends far beyond just selecting the equipment. They contribute to every single aspect that enables the healthcare facility to run smoothly and deliver efficient, quality patient care.
Inventory Management Software: The Future of Successful Equipment Planning
Effective inventory management is a cornerstone of successful medical equipment planning. Inventory management software plays a vital role in ensuring that healthcare facilities have the right equipment and supplies available when needed. This software provides real-time tracking of inventory levels, enabling automatic reordering to prevent stockouts or overstock situations. It also integrates with supply chain management systems to streamline procurement and distribution processes.
By reducing waste, minimizing storage costs, and ensuring the timely availability of essential equipment, inventory management software helps healthcare facilities maintain operational efficiency and deliver high-quality patient care.
Barcoding
The healthcare industry utilizes barcode technology in several ways. As Recommendations for Using Barcode in Hospital Process from the National Institutes of Health (NIH) reports, “Barcode technology can prevent medical errors by providing detailed and reliable information in the site of patient care.”
For success with barcode technology in medical equipment, there are two basic requirements: The barcodes must be well made and clear, and staff must be equipped with the proper scanning technology to read these barcodes. Utilize the latest in medical and pharmaceutical barcode printing and scanning technologies to be competitive in this market.
Medical Equipment Planning and Regulatory Compliance
Meeting regulatory standards forms one of the most crucial steps in medical equipment planning. These include serialization, barcoding, and FDB compatibility. Failing to meet requirements can lead to significant legal issues and compromise patient safety. Here are some essential considerations when it comes to medical equipment planning and regulatory compliance:
1. Understanding Regulatory Standards
The first step towards compliant medical equipment planning is to understand the regulatory standards applicable to your region or country. This might involve directives, regulations, or standards set by entities like the FDA (in the US) and similar bodies globally. Knowing these standards forms the backdrop for compliant planning.
The United States Pharmacopeia (USP) provides annually updated standards for manufacturers and regulatory organizations. These include USP 797 Pharmaceutical Compounding of Sterile Preparations and USP 800 Hazardous Drugs Handling in Healthcare Settings. The current USP 797 and 800 guidelines for pharmacy and/or medical equipment planning are informational concerning compliance with the FDA.
2. Choosing Compliant Equipment
When selecting medical equipment, including medication packaging systems, it is vital to ensure they meet set regulatory standards. Equipment manufacturers will typically provide certifications or proof of meeting these requirements.
3. Integration and Installation Compliance
The way equipment is installed and integrated within your healthcare facility also needs to comply with certain regulations. This could involve everything from ensuring appropriate spatial arrangements to adhering to safety protocols during installation.
4. Training and Usage
Regulatory standards also extend to the correct usage of medical equipment. This dictates the need for trained personnel who understand the medical device operation and comply with usage regulations.
Training for installing equipment, along with safety training and instruction on how to use the equipment, should be made available to purchasing customers.
5. Disposal
When medical equipment reaches the end of its lifespan, compliant disposal methods should be considered in the equipment plan. There are specific rules regarding medical waste disposal, particularly for equipment that contains hazardous materials.
In the grand scheme of things, the role of an efficient medical equipment planner extends to ensuring that every stage in the process, from selection to disposal, follows the regulatory norms put in place.
The Role of Medical Packaging Inc. in Medical Equipment Planning
Medical Packaging Inc. (MPI), is a leading global manufacturer of unit dose pharmaceutical packaging and labeling systems. MPI takes pride in delivering high-quality products and custom-tailored solutions according to the needs of healthcare facilities.
We prioritize the unique needs of our clients, starting from independent pharmacies to specialty pharmaceutical manufacturers and even hospital equipment and surgery centers in need of packaging solutions. Our wide range of products, including unit dose packaging machines and barcode labelers, facilitate the effective packaging and labeling of varying medication forms.
The correct packaging and labeling of medication are crucial elements of patient care. A typical healthcare facility oversees hundreds if not thousands of different medications, each with specific storage and usage instructions. Practical solutions from MPI help these facilities streamline their medication handling processes, reduce errors, and enhance patient safety.
The need for such equipment becomes even more pronounced in certain scenarios – for example, extended care and long-term care pharmacies often have to package oral medications in seven-day or fewer cycles to maintain compliance with the CMS-mandated short-cycle dispensing model. MPI’s specially designed labeling and packaging solutions facilitate such operations.
MPI’s pharmaceutical packaging equipment uses state-of-the-art medical technology to ensure that medication packaging is up to FDA standards. Our easy-to-use and powerful software, Pak-EDGE®, allows pharmacists and techs to design customized and readable barcode labels for each medication.
Contact us or request a free quote to see how we can help your medical facility with its equipment planning needs.
Resources:
“Pharmaceutical Compounding – Sterile Preparations.” USP, www.usp.org/compounding/general-chapter-797. Accessed 26 Aug. 2024.
“Hazardous Drugs-Handling in Healthcare Settings.” USP, www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare. Accessed 28 Aug. 2024.
USP-Chapters-797-and-800-New-and-Revised-Compounding …, www.aha.org/system/files/media/file/2020/02/usp-chapters-797-and-800-new-and-revised-compounding-standards-3_1.pdf. Accessed 3 Sept. 2024.
Hachesu, Peyman Rezaei, et al. “Recommendations for Using Barcode in Hospital Process.” Acta Informatica Medica : AIM : Journal of the Society for Medical Informatics of Bosnia & Herzegovina : Casopis Drustva Za Medicinsku Informatiku BiH, U.S. National Library of Medicine, June 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC4949022/.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients