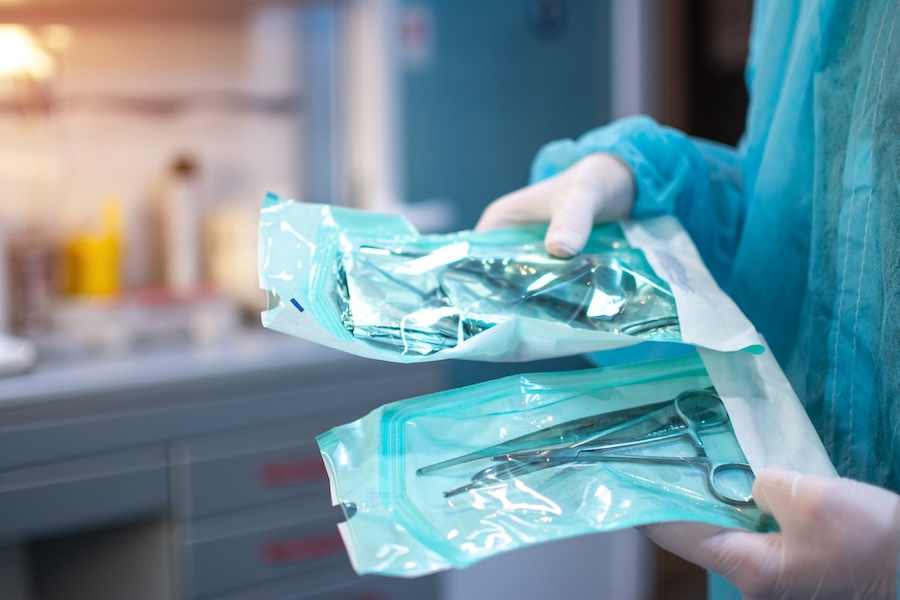
What is Sterile Packaging?
Sterile packaging is a critical component in the healthcare industry. But what exactly does it mean? Quite simply, sterile packaging involves placing medical devices or pharmaceutical products into packages that prevent contamination by microorganisms. The packaging not only has to be a microbial barrier but must maintain its integrity and sterility for an extended period until it is used or the expiration date.
The importance of sterile packaging cannot be overstated. In a healthcare environment, the maintenance of sterility in medical devices and pharmaceuticals is crucial to patient safety. Contaminated instruments or medicines can have serious health implications, including severe infections, adverse reactions, and in some cases, lead to life-threatening situations.
Sterile packaging plays an integral role in reducing these risks by maintaining a sterile barrier from the point of manufacture to the point of use. It encompasses a complex process of sterilizing, packaging, and finally sealing the medical device or medicine in a container that will not breach sterility unless physically opened or damaged.
Processes to Ensure Sterile Packaging
Creating sterile packaging involves a detailed, multi-step process, far beyond simply sealing a product in a bag. The key steps focusing on guaranteeing sterility until the point of use include:
1. Sterilization
This initial step involves eliminating all forms of microbial organisms from the products. Several sterilization methods exist, including:
- Heat Sterilization: This involves subjecting items to high temperatures to kill germs. Effective for many medical products, but potential damage may occur for heat-sensitive materials.
- Ethylene Oxide Gas Sterilization: A preferred method for temperature-sensitive medical devices and instruments, ensuring they remain undamaged during the sterilization process.
2. Monitoring Sterilization Success
Biological and chemical indicators play a crucial role in guaranteeing successful sterilization. Biological indicators, usually containing resilient bacterial spores, symbolize successful sterilization if inactivated. Chemical indicators similarly reflect successful sterilization through physical or chemical changes, like color transitions.
3. Packaging
Post sterilization, keeping the medical devices or pharmaceutical products sterile is the challenge. Here are some key considerations for successful sterile packaging include:
- Shelf Life: The packaging must maintain sterility for the expected shelf life of the product.
- Storage Conditions: Packaging must withstand varying environmental & storage conditions without compromising the sterile barrier.
- Packaging Material: Selected material must prevent microbial contamination while still being safe, compliant, and easy to open.
In summary, the sterile packaging process is well-planned and meticulous, going beyond merely placing a product in a bag. From sterilization to unwrapping, every step is thoughtfully executed to prioritize patient safety and uphold the highest quality standards.
The Importance of Quality & Sterile Packaging in The Healthcare Industry
In the world of healthcare, sterile packaging holds immense importance. It not only impacts various elements like patient safety but also plays a key role in disease prevention and control.
Patient Safety
At the core of patient safety is managing healthcare-associated infections, which can arise from a variety of sources, including the use of non-sterilized medical device packaging. These infections may significantly hamper a patient’s recovery process, extend hospital stays, leave healthcare providers liable, and potentially lead to life-threatening scenarios.
Disease Prevention & Control
Beyond patient safety, sterile packaging significantly contributes to disease control within healthcare settings. With the increasing prevalence of infectious diseases, the assurance of sterile medical devices for healthcare professionals is vital. Effective sterile packaging helps uphold these sterility standards, enabling healthcare providers to deliver quality care without the risk of spreading infections.
Product Protection
The role of sterile packaging extends beyond just eliminating bacteria. It involves protecting complex and often delicate instruments and medications from potential damage during handling, storage, and transportation. This is key to maintaining the functionality and longevity of sensitive medical equipment, which are typically high in cost and complex to repair or replace.
Sterile packaging serves as a crucial pillar in the healthcare industry. It influences more than just immediate patient safety – it directly impacts operational efficiency in healthcare facilities and contributes to the broader goal of effective healthcare delivery and disease prevention.
Essential Packaging Components for Effective Pharmaceutical Packaging
In addition to the crucial role of sterility, several other specific requirements contribute to creating reliable and efficient packaging that ensures product safety, regulatory compliance, and ease of use:
- Clear labeling: Effective medication packaging includes easily visible and comprehensive labeling that displays important information, such as drug details, expiration date, batch number, dosage instructions, and warnings. This ensures both healthcare providers and patients can accurately identify and administer the product.
- Barcodes: Packaging should incorporate barcodes for efficient tracking and inventory management. Barcodes also play a vital role in enhancing patient safety by reducing the chance of medication errors through automatic identification and verification systems.
- Secondary packaging: Secondary packaging and tertiary packaging must be strong enough to withstand handling, transport, and storage conditions without damage. It should protect the contents from environmental factors like moisture, light, heat, or physical stress, ensuring the product arrives intact and functional.
- Tamper-evident seals: Packaging should include tamper-evident features, such as seals or shrink wrapping, to ensure the product has not been compromised before it reaches the end user. This enhances product security and consumer confidence.
- Ease of opening: While security is key, packaging should also be designed for easy and safe opening, especially for elderly or disabled patients. This balance between safety and accessibility is crucial for patient satisfaction and compliance.
Sterile Packaging in the Pharmaceutical Sector
Sterile packaging is not exclusive to medical devices. It plays a significant role in the pharmaceutical sector as well, helping to maintain the integrity of medicines and ensure their safe delivery to patients.
Protection from contamination is a critical aspect of pharmaceutical packaging. Medications are susceptible to spoilage or loss of potency when exposed to environmental factors such as moisture, light, and unclean surfaces. Sterile packaging solutions provide a shield against these hazards, protecting the product’s efficacy until it reaches the consumer.
Supply Chain
The role of sterile packaging extends to ensuring a secure pharmaceutical supply chain as well. Pharmaceuticals often travel long distances and pass-through multiple hands before reaching the end-user. Sterile packaging ensures that these products remain contamination-free throughout this journey, guaranteeing their safety and efficacy at the point of use. Avoiding recalls has never been more important with the rising demand for healthcare products and the current drug shortages.
Compliance
In an industry expected to meet stringent regulations and high-quality standards, pharmaceutical manufacturers heavily rely on robust, sterile packaging options. Issues with packaging sterility can lead to product recalls, regulatory sanctions, and damage to a company’s reputation, making the requirement for reliable sterile packaging solutions a non-negotiable essential.
How Medical Packaging Inc. Contributes to the Sterile Packaging Industry
In the healthcare industry, maintaining the highest levels of safety and quality is critical, particularly when it comes to the packaging of medications. Medical Packaging Inc. (MPI) is proud to offer a range of sterile packaging solutions designed to meet these standards, ensuring patient safety and reducing the risk of medication errors.
MPI’s sterile medication packaging products offer reliable solutions for various healthcare settings. From unit dose packaging solutions that preserve the potency of medications to sophisticated barcode software packaging systems that ensure the organization and inventory management of a facility, MPI takes a comprehensive approach to sterile packaging.
High-Performance Sterile Packaging Equipment
Our sterile packaging equipment is designed for easy operation while delivering top-tier performance. MPI offers unit dose packaging machines for both oral solid and liquid medications, as well as overwrapping systems that protect products in sterile environments. These solutions ensure the safe storage, handling, and administration of medications in compliance with industry standards.
Advanced Barcode Labeling Systems for Inventory Management
Our barcode labeling systems and software are key to ensuring accurate and compliant labeling of sterile medical products. These products streamline the dispensing process for medications such as ampoules, syringes, and oral medications, enabling healthcare providers to efficiently label and track products while minimizing the risk of errors. MPI’s barcode labeling systems are highly versatile and integrate seamlessly into pharmacy workflows, boosting both accuracy and operational efficiency.
We are the trusted partner in compliant, sterile, and efficient medication packaging solutions. Our commitment to delivering sterile packaging solutions supports healthcare providers in delivering safer, more efficient patient care. By offering innovative technology, ease of use, and exceptional customer support, MPI is at the forefront of improving healthcare packaging standards worldwide.
Contact us today or request a quote to see how we can support your sterile packaging efforts.
Resources
“Other Sterilization Methods.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, www.cdc.gov/infection-control/hcp/disinfection-sterilization/other-sterilization-methods.html. Accessed 1 Oct. 2024.
“Ethylene Oxide ‘Gas’ Sterilization.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, www.cdc.gov/infection-control/hcp/disinfection-sterilization/ethylene-oxide-sterilization.html. Accessed 26 Sep. 2024.
Collins, Amy S. “Preventing Health Care–Associated Infections.” Patient Safety and Quality: An Evidence-Based Handbook for Nurses., U.S. National Library of Medicine, www.ncbi.nlm.nih.gov/books/NBK2683/. Accessed 3 Oct. 2024.
Center for Drug Evaluation and Research. “Carton and Container Labeling Resources.” U.S. Food and Drug Administration, FDA, www.fda.gov/drugs/fdas-labeling-resources-human-prescription-drugs/carton-and-container-labeling-resources. Accessed 3 Oct. 2024.
Content
- Processes to Ensure Sterile Packaging
- The Importance of Quality & Sterile Packaging in The Healthcare Industry
- Essential Packaging Components for Effective Pharmaceutical Packaging
- Sterile Packaging in the Pharmaceutical Sector
- How Medical Packaging Inc. Contributes to the Sterile Packaging Industry
- Resources
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients