Pharmaceutical Supply Chain Management: Key Steps & Challenges
In recent years, supply chain problems have significantly impacted many industries, including the pharmaceutical sector. Addressing these challenges is essential, as the pharmaceutical supply chain is a critical component of patient care. Any disruption in this process can negatively affect patient safety and the healthcare industry.
Understanding the key steps involved in the end-to-end supply chain is crucial for pharmaceutical companies in the United States. By gaining this insight, they can develop effective solutions to mitigate supply chain issues and ensure a steady flow of essential medications.
Key Players in the Pharmaceutical Supply Chain
The pharmaceutical supply chain begins with the research and development process and ends with medication dispensing to consumers. Other key parts include:
- Drug manufacturers
- Packaging companies
- Regulatory agencies
- Wholesale distributors
- Healthcare providers
- Pharmacists
The supply chain process contains several steps that are aimed at ensuring patient safety.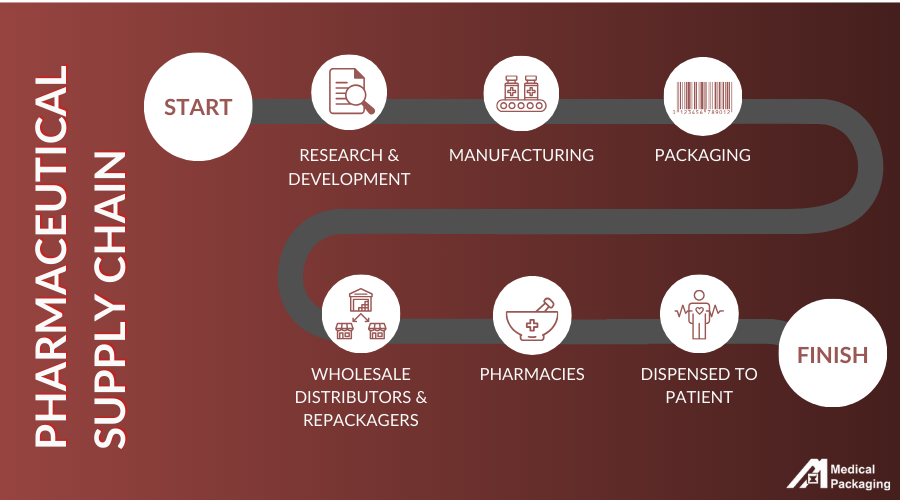
Key Steps Within the Pharmaceutical Supply Chain
The supply chain in the pharmaceutical industry is a complex process due to regulations, safety, and high costs. Overall, each step in the process works to provide individuals access to safe pharmaceutical products, including generic drugs and prescription drugs.
Research and Development
The first step in the pharmaceutical supply chain is Research and Development (R&D), where new drugs are discovered and developed. This process involves identifying potential compounds and conducting initial tests to assess their safety and effectiveness. Successful candidates then move on to more extensive testing with human participants. Throughout this phase, regulatory reviews ensure that safety and efficacy standards are met.
Manufacturing
Pharmaceutical manufacturing is the second major step in the pharmaceutical supply chain. Pharmaceutical manufacturers must have approval from federal agencies, such as the Food and Drug Administration (FDA) before they are allowed to manufacture over-the-counter or prescription drugs for consumers and patients. They utilize raw materials, innovative technology, equipment, and supplies to produce different forms of medications. This includes:
- Tablets
- Capsules
- Liquids
- Creams
- Ointments
- Aerosols
Packaging
Packaging is a key step throughout the pharmaceutical process. Pharmaceutical packaging refers to the materials, designs, and systems that package, store, label, and help distribute the medications to their next destination. Medications must be placed in packaging that meets strict regulations including those set by the Food and Drug Administration (FDA). Packaging must also protect products from damage, such as exposure to light or temperatures. Effective systems and materials can provide real benefits to each key stakeholder in pharmaceutical supply chain management including:
- Reduction of medication errors
- Protection of valuable product
- Compliance with regulatory authorities
- Traceability
- Cost Reduction
Wholesale Distributors & Repackagers
Wholesale distributors or repackagers receive pharmaceutical products from drug manufacturing companies. Wholesale distributors will purchase drugs from manufacturers to store and distribute to healthcare markets. According to the Common Wealth Fund, about 92% of prescription drugs in the U.S. are distributed through wholesalers. A repackager will purchase large amounts of generic drugs and repackage the product into smaller quantities before reselling it in healthcare markets.
Problems with this part of the supply chain, from staffing to equipment to materials can result in delays in pharmacies being able to obtain the medications they need to stock.
Pharmacies
Pharmacies are typically one of the last steps before the drug reaches the consumer. The specific process of distribution will differ depending on the type of pharmacy and the healthcare market where the pharmacy operates.
Whether in hospitals, acute care facilities, integrated delivery networks, long-term care facilities, or specialty markets, pharmacies must get the right medication to the right patient at the right time.
Dispensed to Consumers
The last part of the supply chain is having medications dispensed to consumers. This might involve having consumers purchase prescription medications from healthcare providers or pharmacies. In other cases, consumers can purchase over-the-counter medications from drugstores, supermarkets, department stores, and other retail businesses.
Addressing Challenges in the Pharmaceutical Supply Chain
The pharmaceutical supply chain faces several challenges, from drug shortages to increased drug costs. Taking a closer look at a few of the key challenges in the supply chain can help pharmaceutical manufacturers, packaging companies, distributors, and pharmacies determine efficient, cost-effective solutions.
Costs
The pharmaceutical supply chain can impact the cost of pharmaceutical products. Complexities within the supply chain can lead to higher costs for every stakeholder, including consumers. Finding cost-effective solutions and innovative technology are a few ways to simplify the supply chain and improve operational efficiency to help reduce the cost of medications and drug production.
Regulations
Companies and organizations that are part of the pharmaceutical supply chain often have multiple regulations to meet for safety purposes. Although these regulations are an important part of patient safety in the U.S., complying with them can result in delays or other issues that disrupt the supply chain. Utilizing systems and partnerships that provide speed-to-market regulatory and technical support is one way to speed up the process.
Safety & Security
Safety is the most important element of the supply chain. Drug manufacturing companies are required to ensure product safety and quality during the manufacturing process. Packaging solutions play a vital role in health systems to ensure that medications avoid contamination or medication errors. Utilizing effective packaging and labeling solutions is key for manufacturers, repackagers, wholesalers, and pharmacies to provide safe and traceable medications.
Drug Shortages
Drug shortages are among the biggest supply chain challenges. Without adequate amounts of certain medications to meet customer and patient demands, consumers face difficulties in treating health issues. Pharma companies that can find effective solutions to these shortages, as well as ways to prevent them from happening, can end up having a distinct competitive advantage over other drug manufacturers.
Why Drug Shortages Happen
Drug shortages in the U.S. or the global supply chain can occur for many reasons.
- Problems with Manufacturing Quality: This is a leading cause of drug shortages and can potentially compromise patient safety.
- Obtaining Raw Materials: Pharmaceutical manufacturers sometimes face difficulties in obtaining the raw materials needed for production. For example, production may be delayed if the active pharmaceutical ingredient is unavailable.
- Limited Production Capacity: Drug shortages can also occur due to limited production capacity or drug discontinuation.
- Increased Demand: Sudden spikes in demand, often due to health crises or seasonal illnesses, can outstrip the supply of certain medications.
Read our blog on 2024 drug shortages for more information.
Pharmaceutical Supply Chain Priorities
Although some supply chain disruptions are difficult to prevent, working toward greater efficiency overall can help minimize their negative impact. Here are recent top priorities from U.S. health service providers and pharma/life sciences executives to improve the supply chain: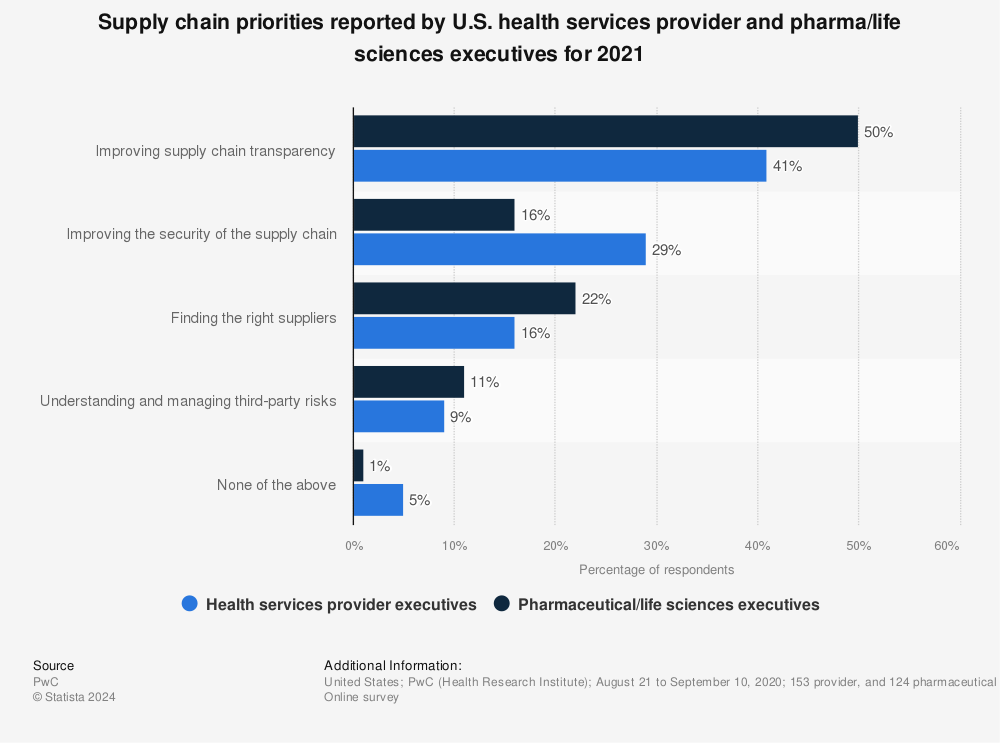
The Future of Supply Chain Management
The future of supply chain management in the pharma industry will be shaped by artificial intelligence and data analytics, driving digital transformation, and enhancing supply chain visibility. These technologies will optimize operations, reduce the carbon footprint, and ensure a more efficient and transparent process from production to delivery.
The Critical Role of Packaging in Pharmaceutical Supply Chain Transparency
Packaging plays a vital role in enhancing the transparency and security of the pharmaceutical supply chain, two main priorities as outlined in the graph above. Packaging ensures that medications are protected from contamination and damage, preserving their efficacy and safety for patients. Clear and accurate labeling on packaging improves traceability, enabling stakeholders to track the journey of a drug from manufacturing to the end user with real-time visibility. This transparency helps in identifying and addressing potential issues quickly, such as recalls or counterfeit products.
Additionally, innovative packaging solutions provide added layers of security and real-time data, further strengthening the supply chain’s integrity. Some key benefits include:
- Tamper-Evident Seals: Protect products from unauthorized access.
- Smart Packaging with Embedded Technology: Offers real-time visibility and tracking data.
- Enhanced Security: Reduces the risk of counterfeit products.
MPI Packaging Solutions for Improved Inventory Management
Medical Packaging Inc., LLC (MPI) offers effective, unit dose packaging solutions for key stakeholders in the pharmaceutical industry. These solutions include different types of packaging systems and materials that are designed to improve safety, promote efficiency, and reduce costs in pharmaceutical supply chain management.
Our offerings include oral solid packaging systems, labeling solutions, overwrapping systems, oral liquid packaging systems, medical packaging materials, and more.
Our completion of a Type III Drug Master File (DMF) submission to the U.S. Food & Drug Administration (FDA) allows us to ensure a rapid response to customers’ compliance and filing needs within the pharmaceutical industry. It also helps us meet the needs of pharmaceutical drug manufacturers and contract drug manufacturing organizations (CDMOs) for FDA-compliant liquid cup packaging capabilities.
For more information on MPI’s packaging solutions, please contact us.
Resources
Mikulic, Matej. “Supply Chain Priorities of Provider and Pharma/Life Sciences Executives U.S. 2021.” Statista, 26 Jan. 2021, www.statista.com/statistics/1196032/supply-chain-priorities-health-services-provider-and-pharma-executives/.
“The Impact of Pharmaceutical Wholesalers on U.S. Drug Spending.” Commonwealth Fund, 20 July 2022, www.commonwealthfund.org/publications/issue-briefs/2022/jul/impact-pharmaceutical-wholesalers-drug-spending.
Content
- Key Players in the Pharmaceutical Supply Chain
- Key Steps Within the Pharmaceutical Supply Chain
- Addressing Challenges in the Pharmaceutical Supply Chain
- Drug Shortages
- Pharmaceutical Supply Chain Priorities
- The Critical Role of Packaging in Pharmaceutical Supply Chain Transparency
- MPI Packaging Solutions for Improved Inventory Management
- Resources
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients
