Understanding The Unit Dose Supply Method

Drug administration is a high-risk practice in and of itself. Aside from that, medical personnel in healthcare facilities are under extreme time constraints. Regardless, the only thing that matters, in the end, is that the correct medication at the proper dose reaches the right patient at the right time.
As a result, using the unit dose system helps hospitals reduce medication errors while also saving time for patient care.
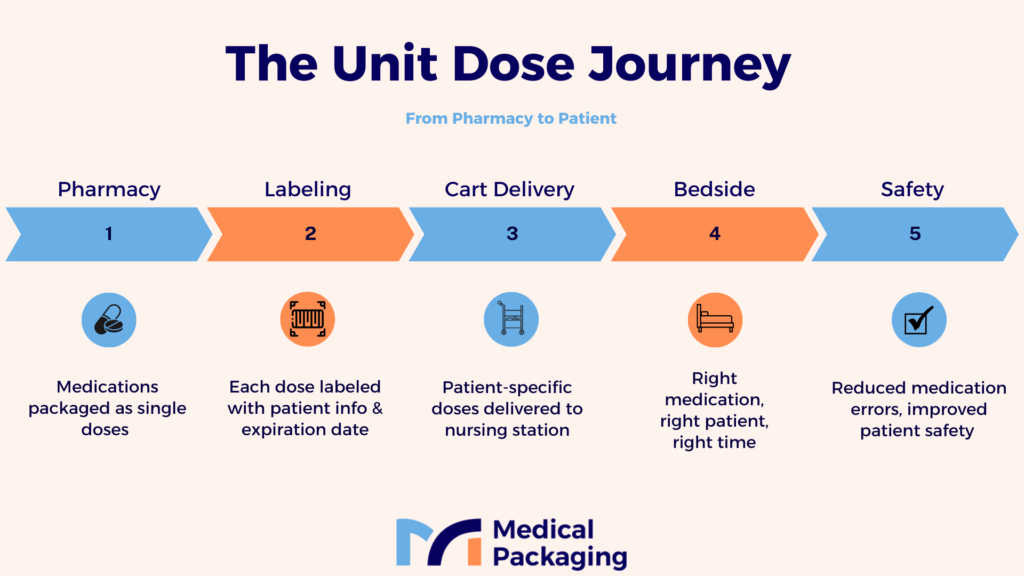
Enhanced Patient Safety
The unit dose supply method delivers the recommended dose of a given medicine to a specific patient at the time of administration. It differs from previous approaches in that each dose of prescription medicine is packaged separately in a ready-to-administer form as a single dose.
Each unit dose container is labeled so that it can be identified until it is administered to the patient. The packaging, which has a label showing detailed information, including the drug name, strength, control number, and expiration date, minimizes contamination caused by the drug product’s transfer and handling. As a result, this patient medication package approach significantly decreases the possibility of medication errors and enhances medication safety.
Operational Benefits
The unit dose method delivers enhanced drug control and monitoring while reducing medication waste and pilferage. Floor stock requirements decrease significantly, and pharmacists gain better oversight of work schedules and patient medication profiles.
Staff Efficiency
By centralizing dose preparation in the pharmacy, nurses and pharmacists spend more time on direct patient care rather than medication handling tasks. This improved utilization of hospital staff directly impacts patient outcomes.
Financial Advantages
The system reduces revenue losses through accurate billing – patients pay only for medications administered to them. Credit returns for unused medications decrease, and inventory management becomes more precise with 24-hour patient-specific dosing.
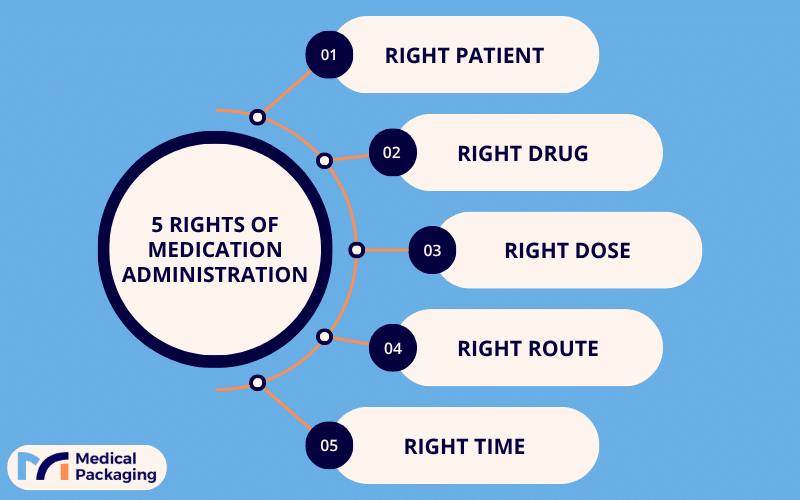
It is a system that supports the five rights of medication administration:
- Right patient
- Right drug
- Right dose
- Right route
- Right time
Planning a unit dose system is an uncomplicated process that can be done without interrupting the pharmacy operation or impacting patient care.
Identify Medications to Package in Unit Dose Form
The process starts with the hospital identifying all medications for use as a unit dose drug and determining the form of packaging needed. The unit dose method is best suited for medications that come in bulk packaging.
However, most other types of drug packaging, such as suppositories, ampoules, transdermal patches, inhalation solutions, or prefilled syringes, can be packaged into unit dosages. After creating a unit medicine dosage, the oral solids or liquids can be ordered from the hospital pharmacy for safe medication administration.
One significant advantage of unit dosages is that the pharmaceuticals are not removed from the unit dose packaging until they reach the patient. This also implies no cross-contamination with other medications or human contact, particularly important when managing controlled substances.
Unit dose tablets and other dosage forms can also be used to develop a patient-specific treatment that incorporates more than one medication. The medications are delivered to nursing stations in patient care areas through patient-specific treatment carriers. This approach drastically reduces the time nursing personnel spend physically storing, sorting, and providing medication. Furthermore, clear labeling of medication, dose, batch, and expiration date improves safety in handling high-risk medications. The use of barcoded unit dose products and scanning procedures also helps with drug identification and supports the drug distribution system while ensuring patient safety.

Facility Considerations
Type of Packaging Option for Each Medication
After the healthcare facility determines which medications are best suited for this unit dose supply method, and the ideal delivery method necessary for each, they must go through a system selection process that includes identifying the necessary equipment.
When identifying equipment requirements, include packaging equipment for the various dosage forms of medications such as tablets, capsules, injectables, and liquids. Consider whether medications require specialized container solutions such as foil pouch packaging for enhanced stability. Other considerations include:
- Floor carts for direct distribution
- Modular filling stations
- Patient profile holders
Another critical decision to make is to decide what items should be purchased in unit dose packages, what is best for in-house repackaging, and how that is accomplished. This will involve identifying the unique package requirements, what supplies the pharmacy needs, and what volume is necessary for each format. The pharmaceutical industry continues to evolve toward more automated solutions for both long-term care facilities and acute care settings.
Unit Dose Budgeting
When budgeting for the unit dose supply method, the administration must determine the cost of the equipment and consumable supplies and the potential return on investment. How much is implementing this system saving the hospital?
Implementation and Training Considerations
Also, consider the impact the implementation of unit dose packaging will have on the staff and patient care workflow. How will it affect different pharmacy or hospital settings? For example, the impact will be different in the pediatric wards than in the areas that deal primarily with adult patients, such as an acute care facility or long-term care facility.
The administration will also need to consider training periods when planning implementation. Training will start in the pharmacy and then move to the medical and nursing units. Training can be conducted in a way that will not disrupt patient care.
Some efficient training approaches might include:
- Rotating pharmacy staff to work on the single dose dispensing every week until everyone goes through the training process
- Rotating the nurses through audiovisual presentations of the new system
- Providing general staff with information on the drug administration changes via newsletters or media
Training small groups at a time is the most effective way to avoid interruptions in patient care while still ensuring all employees understand the unit dose supply method. Each nurse and healthcare professional needs proper documentation and education on the new procedures.
At Medical Packaging Inc., LLC (MPI), we provide services to support clients not just when they are first establishing their unit dose system program but throughout training to implementation to utilization.
Establish Internal Planning for All Unit Dose Packaged Medications
The unit dose supply method requires careful planning before implementation beyond equipment, supplies, and training. In addition, protocols for dose preparation need to follow safe medication practices closely and align with administrative orders from healthcare providers.
The healthcare facility will also need to have a system in place for storage, whether it is in a bulk storage space or on a cart in the nursing unit. There must also be plans for internal flow from patient admission to patient care area to patient medication profile to packaging to filling drug delivery carts.
Some common considerations include:
- Setting up the ward stock procedures
- Designing stock batch controls
- Inventory tracking and management
- Determine how to manage drugs not used
- Establishing return authorization number procedures for unused medications
There may also be a need to modify existing procedures and integrate current technology to support the new way of ordering and administering medication, along with how to do proper dose calculations and avoid medication errors.
Additional Pharmacy Requirements to Review
Space Assessment
Conduct a space assessment to see how much room is available and decide how much extra space might be needed. A centralized pharmaceutical storage system with pharmacy automation solutions is essential to allow medical professionals to deal with unit dosages throughout a healthcare facility. Consider requirements for large surfaces needed for packaging operations and storage.
Pharmacy System Reconfiguration
Nurses are liberated from repetitive chores and may dedicate more time to patient-related activities by centralizing drug storage and therapy preparation inside the central pharmacy. Selection and sorting mistakes that occur during the preparation of manual drug distribution are also eliminated. Therapies can be produced at any moment, whether planned or unplanned. The unit dosage drug and the therapeutic ring can also be manufactured at the same time.
It is necessary to ensure compliance with the Consumer Product Safety Commission and industry standards i.e. USP 800 and serialization. At the same time, healthcare facilities must implement safety protocols, QA standards, and testing. Regulation compliance requires proper documentation and stability studies for packaged medications.
However, the most critical aspect of implementing the unit dose supply method is collaborating with the right partner for equipment and packaging needs that can deliver high bond strengths for reliable package integrity.
We are always developing our pharmacy packaging technologies to ensure continuing conformity to industry rules and regulatory requirements for unit dose pharmaceutical packaging.
Future Unit Dose Packaging Trends
Unit dose packaging, especially in the pharmaceutical sector, is expected to see significant growth due to increasing demands for precise dosing and patient safety. Innovations in smart packaging technologies, like RFID and QR codes, will enhance medication adherence and tracking, making unit dose packages more interactive and informative. Additionally, the shift towards AI will become even more apparent in the coming months and years.
A systematic review of current practices shows that facilities implementing proper unit dose systems see significant improvements in medication safety and operational efficiency. The keystone unit dose approach has proven particularly effective in university hospital settings where complex medication regimens are common.
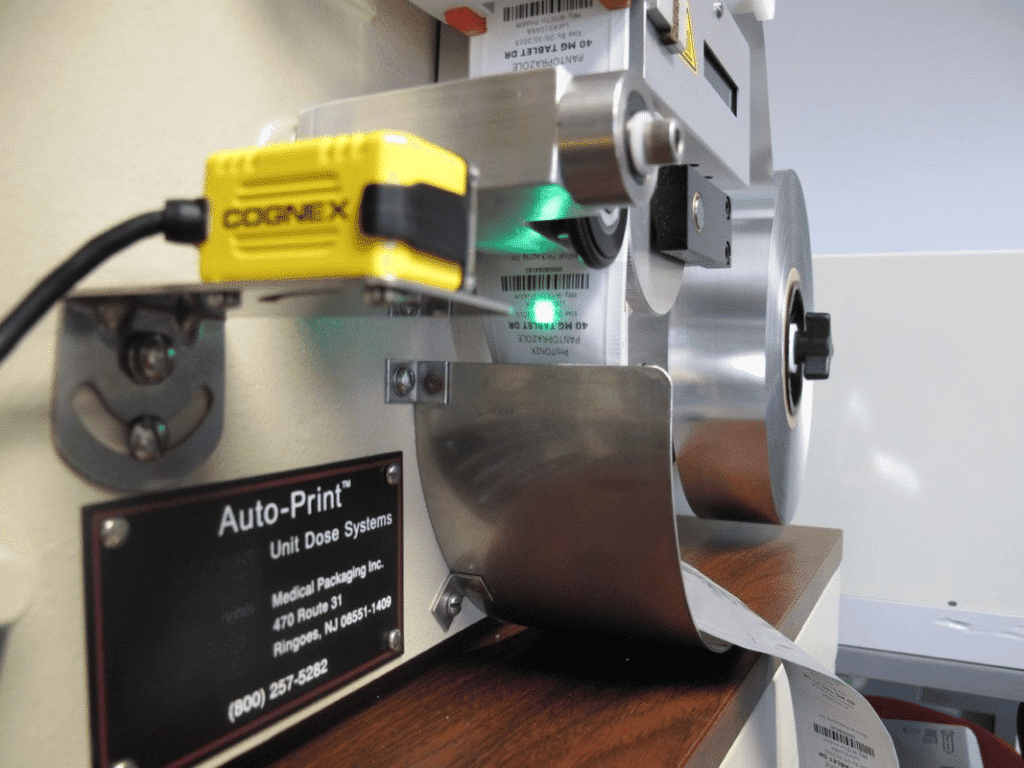
MPI's Role in Unit Dose Packaging
Medical Packaging Inc., LLC was founded in 1971 and has since evolved to become one of the world’s top unit dose packaging system manufacturers. We take pride in having an accepted Drug Master File (DMF), producing high-quality pharmaceutical packaging equipment, MPI-certified consumable materials, and our proprietary Pak-EDGE® UD Barcode Labeling Software Version 2.0.
MPI, headquartered in the United States in Flemington, New Jersey, provides services to customers in North America, Latin and South America, Europe, the Middle East, and emerging countries across the world. MPI aims to be the leading provider of pharmacy packaging solutions that help save patients’ lives while boosting productivity and profitability for all clients.
We provide high-quality items at reasonable costs, and our equipment offers the most up-to-date features and functionality while preserving optimum dependability and simplicity of use. All our clients receive prompt, professional, and courteous customer service and technical assistance. We establish, maintain, and promote continuing connections with our customers, strategic partners, and distributors alike, based on a foundation of professionalism and excellence.
Important Note: This document is for informational purposes only and should not be considered medical advice. Always consult with qualified healthcare professionals for specific medical guidance and recommendations.
Contact us or request a quote to learn more!
Resources
“NFC vs. QR Codes: Everything You Need to Know.” atlasRFIDstore, www.atlasrfidstore.com/rfid-insider/nfc-vs-qr-codes-everything-you-need-to-know/. Accessed 20 March. 2024.
Author links open overlay panelKeisheni Ganeson a, et al. “Smart Packaging − a Pragmatic Solution to Approach Sustainable Food Waste Management.” Food Packaging and Shelf Life, Elsevier, 7 Feb. 2023, www.sciencedirect.com/science/article/abs/pii/S2214289423000212.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients