Medication Repackaging Guidelines: Best Practices for Safety and Efficiency
Prescription drug manufacturers often produce medications on a large scale. Pharmaceutical repackagers then acquire these medications in bulk and repackage them for distribution across diverse pharmacy markets within the healthcare industry.
As part of the drug repackaging application process, the FDA inspects the container in which the medicine will be repacked, as well as the conditions where the repackaging will occur to ensure the quality and consistency of each product. Repackagers must adhere to these strict guidelines to prevent contamination and other medication errors or face fines and legal consequences. 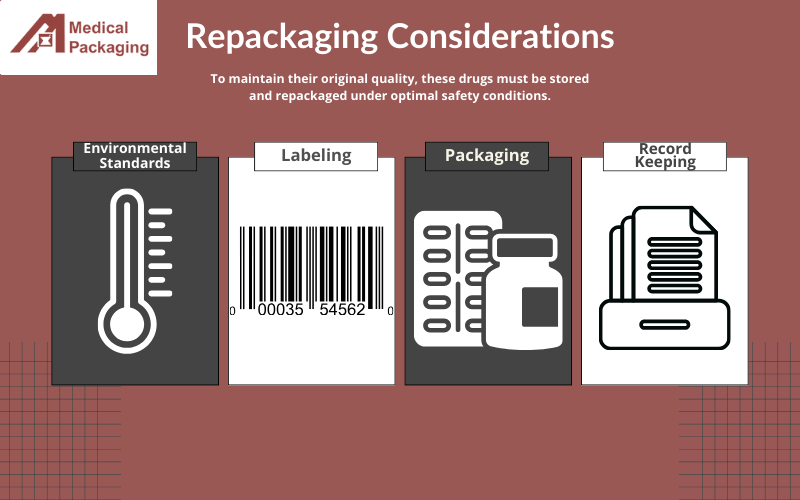
Repackaging Considerations for Traditional Pharmaceuticals
The FDA regards packaging as “the act of taking a finished drug product from the container in which it was distributed by the original manufacturer and placing it into a different container without further manipulation of the drug.” To pass the FDA’s strict standards, repackagers must consider many factors.
Environmental Conditions
To maintain their original quality, these repacked drugs must be stored and repackaged under optimal safety conditions, including:
- Temperature Control
- Humidity and Moisture Control
- Light Exposure
- Air Quality
- Dust and Particles Control
Labeling
Repackagers must follow any instructions on the original drug label for transportation and storage requirements. The new labels for the repackaged drug must contain specific labeling information such as:
- Prescription name
- The name of the repackaging facility (address, phone number, etc.)
- Batch number of repackaged medications
- Date of repackage
- List of ingredients
- Dosage
- Storage conditions
- Handling instructions
- “BUD” (beyond use date)
Packaging
Proper packaging is essential to maintaining the integrity of the medicine. Compliant packaging must have a secure closure system, to ensure that the controlled substance remains sterile throughout the entire process. Low-quality materials could puncture or tear, causing the product to break compliance guidelines. Failure to properly label and package the drugs can result in contamination and product degradation.
Record Keeping
Keeping up-to-date records on medical repackaging is another essential factor to consider. Important records would be the date of repackage, prescription name, the receiving physicians, the quantity of drugs acquired, and more. Records protect repackagers from mistakes and failure to adhere to important guidelines.
Repackaging Biologics: Unique Challenges
As biologics are much more complex than standard medications, their repackagers face alternative challenges when adhering to federal guidelines. Following guidelines for biologics is extremely important as the FDA outlines “Repackaging a drug or biological product could change its characteristics in ways that have not been evaluated during the approval process and that could affect the safety and effectiveness of the product.” Because of their sensitive nature, repackagers must consider their specialized repackaging needs such as:
- Any materials used for mixing or diluting are sterile.
- Biologics must be mixed or diluted in a state-licensed pharmacy.
- Biologic mixing and dilution must be under the direct supervision of a licensed pharmacist.
- Repackaged biological drugs must be shipped and stored in the way the label outlines.
- Biologics must be completely repackaged in no more than 4 hours.
- Repackaged biologics must follow strict documentation of dates and times by which the product should no longer be used.
Cost implications
Following all these guidelines, especially for biologics, can have huge cost implications for organizations. While the initial investment in equipment, training, and process management may be large, overall, it can save costs by avoiding medication errors and legal penalties. 
Regulatory Framework & Compliance
Medication repackaging guidelines are set forth by the FDA. The goal of medication repackaging guidelines is to ensure the safety, efficacy, and quality of medications throughout their repackaging process until they reach the patient. These guidelines are designed to protect patients by maintaining the integrity of pharmaceutical products, despite the change in packaging from the manufacturer’s original to a new format suitable for distribution and use. Key objectives include:
Patient Safety
By adhering to high standards for medication repackaging, healthcare organizations can enhance patient trust and satisfaction. Every guideline is put in place to ensure that patients receive safe and quality medicines.
Standardization & Quality Control
Guidelines for medication repackaging support quality control by establishing standardized procedures and criteria for the repackaging process, ensuring that repackaged medications meet the same safety and efficacy standards as those set by the original manufacturers. Because FDA inspection mandates testing, documentation, and adherence to best practices the risks of contamination, degradation, or errors are minimized.
Reduce Error
These guidelines prevent mix-ups, incorrect dosing, and other mistakes, enhancing patient safety and treatment efficacy.
Consumer Confidence
Patients are more likely to have confidence in the safety and quality of their medications, which can contribute to better health outcomes and loyalty to healthcare providers.
Classifications of Repackaging
There are three different tiers to pharmaceutical packaging that classify each layer differently. All three layers must work together to meet guidelines and protect the product within.
Primary Packaging
Primary packaging involves the material that touches the product. These directly affect shelf life and come in the forms of:
- Ampoules
- Vials
- Syringes
- IV Bag
- Pill Bottles
- Blister packs
Medical Packaging Inc., LLC (MPI) offers FDA-approved primary unit dose repackaging for oral solid medication and oral liquid medication. Along with these primary packaging solutions, we also offer labeling accessories like our barcode software Pak-EDGE®.
Secondary Packaging
Secondary packaging is the system that overwraps primary packaging and comes in many forms such as:
- Cartons
- Boxes
- Overwrapping
- Injection Trays
MPI is proud to offer top-tier secondary packaging solutions as well as primary. Our Pharmacy Accessory Bagging System (PABS) serves as an FDA-approved overwrapping solution for ampoules, vials, and syringes.
Tertiary Packaging
Tertiary packaging is used for bulk medicine handling and shipping. They are typically used as:
- Barrels
- Containers
- Edge Protectors
It is important to note that although tertiary packaging does not directly touch the product, it must follow strict guidelines to ensure efficacy.
Labeling Guidelines
Labels on repackaged products must identify the drug name, strength, dose, and other essential information. By tracking medications through barcodes, it is easier to manage recalls and trace adverse reactions back to specific batches of medications. This traceability is critical for patient safety and regulatory affairs. Barcodes enable more accurate and efficient inventory control, reducing waste and ensuring that medications are available when needed. They help in monitoring stock levels, expiration dates, and managing reorders.
MPI’s barcode labeling software Pak-EDGE® is a customizable labeling program for medical and pharmaceutical markets. This advanced labeling program provides a single user interface to all MPI packaging systems, included in the purchase of a new packaging system.
The Future of Repackaging
The landscape of medication packaging is undergoing transformative changes, driven by a focus on enhancing patient engagement and compliance. The shift towards unit dose containers is gaining traction for its ability to simplify dosage understanding and adherence for patients. The integration of automated dispensing systems is streamlining the medication dispensing process, offering a more accurate and efficient way to manage patient prescriptions and reduce potential drug mix-ups. Additionally, the advent of on-the-spot medication dispensing in urgent care settings is eliminating delays in treatment initiation, allowing patients immediate access to necessary medications.
Innovations in smart packaging technology are at the forefront of this evolution, enabling real-time tracking of medication adherence through sophisticated tablet blister packaging equipped with electronic monitoring and communication capabilities. This not only enhances patient safety by alerting healthcare providers to compliance issues but also contributes to a more personalized approach to medication management.
The role of packaging and delivery systems in distinguishing pharmaceutical products is becoming increasingly critical. To address challenges such as drug counterfeiting and ensure supply chain integrity, the industry is embracing packaging solutions that offer advanced security features, including track-and-trace technologies and product authentication. Furthermore, according to The National Library of Medicine, material science innovations are paving the way for cleaner and more efficient packaging options, such as advanced elastomeric formulations and barrier coatings, promising to uphold the highest standards of purity and safety in medication packaging.
As new and innovative treatments emerge within the pharmaceutical industry, the way medications are packaged and delivered is advancing right alongside. Leveraging the latest in artificial intelligence, design, and material science, the future of medication packaging is set to significantly improve the safety, effectiveness, and ease of use for people all around the globe.
MPI Provides Safe Repackaging Options for All Customers
We offer a full range of medication packaging equipment that adheres to strict FDA standards for customers in a variety of pharmaceutical services. This allows customers to put their full trust in our technologies to deliver optimal results.
Medical Packaging Inc., LLC (MPI) has proudly achieved a milestone with the acceptance of a Type III Drug Master File. This specific DMF number relates to packaging materials, underscoring MPI’s commitment to helping our partners meet guidelines and bring their offerings to market. We disclosed every packaging material, component, intended usage, as well as manufacturer information to demonstrate our product quality assurance.
Our products help repackagers and other markets follow all repackaging guidelines. Our goal is to simplify your pharmacy processes with the most advanced technology in the industry. Our expertise allows us to recommend products that will best suit the needs of your industry and promote overall efficiency.
Contact us today to learn more about our compliant pharmacy packaging and labeling products.
References
Repackaging of Certain Human Drug Products By …, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Jan. 2017, www.fda.gov/media/90978/download.
Admin. “Repackaging Medication: What Is It? – Proficient Rx.” ProficientRX, 16 Nov. 2023, proficientrx.com/repackaging-medication-how/.
Mixing, Diluting, or Repackaging Biological Products …, www.fda.gov/files/drugs/published/Mixing–Diluting–or-Repackaging-Biological-Products-Outside-the-Scope-of-an-Approved-Biologics-License-Application.pdf. Accessed 22 Feb. 2024.
Zadbuke, Nityanand, et al. “Recent Trends and Future of Pharmaceutical Packaging Technology.” Journal of Pharmacy & Bioallied Sciences, U.S. National Library of Medicine, Apr. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3697200/.
Antwerp, George Van, et al. “The Pharmacist of the Future.” Deloitte Insights, Deloitte, 6 June 2023, www2.deloitte.com/us/en/insights/industry/health-care/future-of-pharmacists.html.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients
