The Future of AI in Healthcare: Advanced Pharma Manufacturing, Limitations, & Precision Medicine
As medicine continues to evolve, artificial intelligence is taking center stage, transforming healthcare and drug development. The integration of AI technology in the pharmaceutical industry marks a significant shift towards more efficient, accurate, and innovative practices, particularly in drug discovery and patient care.
By harnessing the power of machine learning, generative AI, and predictive analytics, the pharmaceutical sector can expedite the drug development process, from initial discovery to clinical trials, while minimizing the potential for human error and bias. This approach to real-world applications not only accelerates the pace at which new drug candidates reach patients but also provides a higher degree of precision and personalization in healthcare delivery. As AI algorithms continue to advance, their application within the pharma industry promises to unlock new horizons in the treatment of complex diseases, reshaping the healthcare landscape for the better.
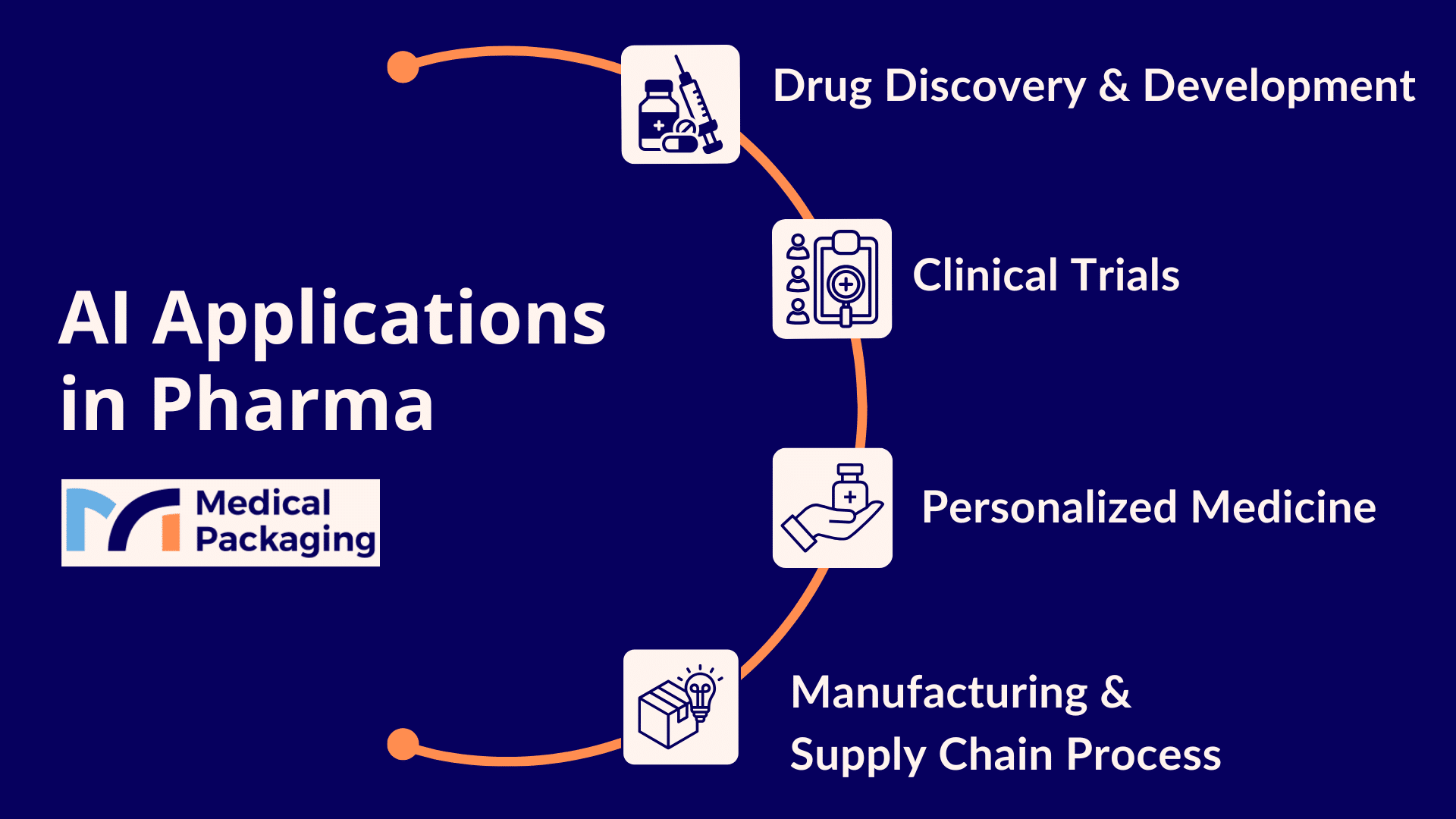
Drug Discovery & Development: The AI Revolution in Medicine
Traditionally, the drug discovery process has been a lengthy, complex, and costly endeavor with a high rate of failure. However, AI-powered platforms are now able to rapidly analyze vast datasets of chemical compounds, patient data, and protein structures to identify potential drug candidates. AI tools like generative AI and deep learning algorithms can simulate and predict how different compounds will interact with biological targets, significantly reducing the time and resources required to identify viable drug candidates.
AI-Driven Breakthrough Examples
Much of this is already underway as Exscientia introduced their first AI-designed drug molecule for OCD patients to enter human clinical trials in early 2020. More recently, pharmaceutical companies have leveraged AI systems to analyze unstructured data from diverse sources, including genetic information and sensor data, to accelerate molecular design processes.
Target Identification and Validation
The initial step in the development process usually involves identifying biological targets that play pivotal roles in disease pathways. AI applications, particularly through machine learning and deep learning, excel in processing complex biological data science to uncover potential targets. By analyzing genetic information from vast amounts of patient data, AI algorithms can predict which proteins or genes are implicated in a disease and therefore represent promising targets for new pharmaceutical products.
Drug Property Prediction
Life sciences companies are utilizing AI technology to predict drug properties through automated analysis:
- Toxicity Assessment: AI models analyze molecular structures to predict potential toxic effects before physical testing
- Bioactivity Prediction: Machine learning algorithms assess how effectively compounds interact with biological targets
- Physicochemical Characteristics: AI systems evaluate drug absorption, distribution, metabolism, and excretion properties
- Automated Testing: AI reduces the need for physical testing in many scenarios by running completely automated virtual tests
The pharmaceutical sector benefits from AI’s ability to process vast datasets more efficiently than traditional methods.
Future Impact on the Pharmaceutical Industry
Because AI-powered drug discovery can significantly reduce the timeline for development, more medicines will be produced at higher rates, opening opportunities for this growing pharma industry. The future application of AI can increase drug accessibility, advance pharmaceutical research, and further fuel this billion-dollar pharmaceutical sector.
AI in Clinical Trials
The integration of artificial intelligence into clinical trials is setting new standards for efficiency, safety, and patient-focused design in the pharmaceutical industry. Recent research shows AI is reshaping trial design, patient recruitment, and outcome prediction, helping reduce risk and improve success rates. By applying AI to large pharmaceutical datasets, companies can better identify promising drug candidates, accelerate development timelines, and improve the speed of regulatory approvals.
Patient Selection
One of the most important aspects of clinical trials is identifying and enrolling patients who are most likely to benefit from the investigational treatment. AI algorithms excel in analyzing vast amounts of data from patient charts, electronic health records, and other diverse sources to match patients with clinical trials for which they are best suited. This precision in patient recruitment not only improves the chances of success for clinical trials but also allows patients to receive treatments more closely aligned with their specific conditions, thereby advancing precision medicine in the pharma industry.
Predicting & Preventing Safety Issues
AI’s predictive analytics capabilities are invaluable for identifying potential safety signals before they manifest. By analyzing historical data from previous clinical trials and real-world evidence, AI systems can forecast safety concerns and adverse effects, allowing researchers to mitigate these risks proactively. This foresight contributes to safer clinical trials and helps maintain study integrity, providing that patient well-being remains paramount.
Patient Monitoring
During the patient monitoring phase, AI technology plays an important role in improving safety and efficacy. Real time data analysis enables early detection of adverse effects, allowing for swift interventions. Additionally, AI can monitor patient compliance and engagement, providing insights that help adjust trial protocols on the fly to improve outcomes. This real time monitoring capability provides a safer trial environment and can lead to more conclusive trial results.
Protocol Generation
Leveraging data analytics from previous trials, AI can quickly generate first-draft protocols that are tailored to specific clinical study goals while incorporating lessons learned from past pharmaceutical research. This rapid protocol generation not only accelerates the trial design phase but also allows for more agile responses to emerging data, speeding up the iterative process of trial optimization.
Accelerating Drug Approval Processes
By improving efficiency at every stage of the clinical trial process, AI has the potential to speed up the overall drug approval process. With faster patient recruitment, improved safety monitoring, and quicker protocol development, trials can reach their endpoints more swiftly. This efficiency gain not only benefits pharma companies by reducing go to market time for new drugs but also has significant implications for patients waiting for novel therapies.
Precision Medicine for The Healthcare Industry
In healthcare, precision medicine is replacing the traditional one-size-fits-all model, thanks to AI advancements. With the increasing complexity of diseases and the unique genetic makeup of individuals, there is a growing need for treatments tailored to each patient. AI is leading this transformation by offering unprecedented capabilities for customizing healthcare at the individual level.
Customizing Treatment Plans with AI
Precision medicine uses a patient’s genetic information, family history, and other key data to create personalized treatment plans. AI excels in analyzing vast datasets, such as genomic data, electronic health records, and biometric information, to identify patterns and predict the most effective treatments for each person. This personalized approach considers unique disease manifestations and responses, going beyond generic treatments.
Improving Drug Safety and Supporting Rare Diseases
AI enhances drug safety by predicting potential adverse reactions based on genetic markers. By analyzing historical and current health data, AI helps identify the right drug combinations and dosages, minimizing trial-and-error. This is especially beneficial for patients with rare diseases, where limited data often hampers effective treatment development. AI can leverage available data to create personalized treatments for even the rarest conditions, offering new possibilities for those with previously limited options.
AI in Manufacturing & Supply Chain Management: Advancing Pharma Operations
The pharmaceutical industry’s manufacturing and supply chain processes are witnessing significant advancement, driven by AI adoption. With the complexities of biopharma production and the need for efficient distribution channels, AI is emerging as an enabler of operational efficiency, cost-effectiveness, and real time decision-making.
Improving Packaging Processes with AI
In pharmaceutical packaging, AI technology is setting new standards for precision, efficiency, and safety. Non-contact packaging methods powered by AI not only protect the integrity of sensitive pharmaceutical products but also significantly reduce contamination risk. These AI-driven systems can manage delicate operations at high speeds, helping pharmacies and manufacturers save both time and money. Additionally, AI facilitates systematic labeling by efficiently managing large sets of data to provide accuracy and regulatory compliance, thereby minimizing error risk.
Supply Chain Management Forecasting
AI enhances demand forecasting in the pharmaceutical industry by analyzing vast amounts of historical data, market trends, and external factors like epidemiological data or healthcare policy changes. This enables more accurate predictions, helping companies optimize production schedules, manage inventory, and allocate resources effectively, leading to a more resilient supply chain.
Optimizing Equipment Performance and Maintenance
AI plays a significant role in improving operational effectiveness of manufacturing equipment. Using AI algorithms and machine learning, pharmaceutical manufacturers can predict when machines are likely to require maintenance or are at risk of failure. This predictive maintenance approach, informed by continuous monitoring and analysis of equipment data, allows for timely interventions, reducing downtime and extending machinery lifespan. By providing that equipment operates at peak efficiency, AI contributes to smoother production processes and higher product quality control.
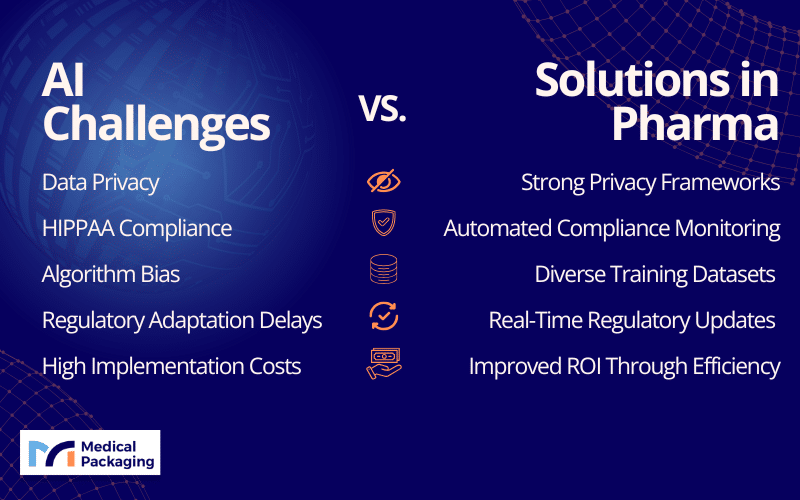
Limitations & Biggest Challenges
Despite its potential, the integration of AI into drug development and discovery faces significant challenges:
- Data Privacy and Compliance: HIPAA violations and data privacy concerns when handling patient information
- Data Requirements: Need for vast datasets from diverse sources to train effective AI models
- Collaboration Complexity: Requirement for interdisciplinary collaboration among scientists, data engineers, and healthcare professionals
- Regulatory Adaptation: Regulatory bodies are still adapting to rapid technological advances, necessitating clear guidelines and standards for AI use in medicine
Ethical and Societal Implications
AI’s role in making important decisions in drug development and patient care raises important ethical questions:
- Algorithmic Bias: Potential for AI systems to perpetuate or amplify existing healthcare disparities
- Flawed Data Risks: AI may recommend treatments based on incomplete or biased datasets
- Automated Decision-Making: Implications of removing human oversight from critical healthcare decisions
- Trust and Oversight: Need for ethical guidelines to maintain trust and uphold the highest standards of patient care
Overcoming the Challenges
Addressing these challenges requires a multifaceted approach:
- Data Privacy Frameworks: Development of thorough privacy protections for patient information
- Regulatory Engagement: Active collaboration with regulatory bodies to clarify regulatory compliance requirements
- Infrastructure Investment: Strategic investments in AI infrastructure and talent development
- Interdisciplinary Collaboration: Fostering cooperation across scientific, technical, and healthcare disciplines
- Ethical Guidelines: Establishment of clear ethical standards to guide AI development and application in the pharmaceutical sector, providing that these technologies benefit society while respecting individual rights and values

Future of AI in Pharmacy with MPI
Medical Packaging Inc., LLC (MPI) is a company ready to move with the shifting AI applications in the pharmaceutical industry. MPI has been at the forefront of innovation in the packaging industry, providing safe, accurate, and efficient medical packaging solutions.
With our proprietary barcode labeling software, Pak-EDGE®, MPI is proud to offer a single-user interface to all of our packaging and labeling systems, giving our customers the tools they need to get the most from their powerful yet cost-effective MPI equipment. Whether you have oral solid, oral liquid, or overwrapping needs, we are prepared to provide you with the most advanced solutions for improved operational efficiency and regulatory compliance.
Contact or request a quote from MPI today for any inquiries surrounding medical packaging for modern, secure packaging solutions that support the evolving needs of the pharmaceutical industry!
References
Sahu, Manisha, et al. “Artificial Intelligence and Machine Learning in Precision Medicine: A Paradigm Shift in Big Data Analysis.” Progress in Molecular Biology and Translational Science, U.S. National Library of Medicine, Apr. 2022, www.ncbi.nlm.nih.gov/pmc/articles/PMC11670736/.
Chun, Matthew. “How Artificial Intelligence Is Revolutionizing Drug Discovery – Bill of Health.” Bill of Health – The Blog of the Petrie-Flom Center at Harvard Law School, 8 Mar. 2023, blog.petrieflom.law.harvard.edu/2023/03/20/how-artificial-intelligence-is-revolutionizing-drug-discovery/.
Paul, Debleena, et al. “Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine.” U.S. National Library of Medicine, Oct. 2024, www.ncbi.nlm.nih.gov/pmc/articles/PMC11510778/.
Suriyaamporn, Patcharaporn, et al. “The Artificial Intelligence-Powered New Era in Pharmaceutical Research and Development: A Review.” AAPS PharmSciTech, U.S. National Library of Medicine, Aug. 2024, www.ncbi.nlm.nih.gov/pmc/articles/PMC12156693/.
Johnson, Kevin B, et al. “Precision Medicine, AI, and the Future of Personalized Health Care.” Clinical and Translational Science, U.S. National Library of Medicine, Jan. 2021, www.ncbi.nlm.nih.gov/pmc/articles/PMC7877825/.
Austin, Christopher P. “The Revolution of Personalized Medicine Is Already upon Us in Rare Diseases.” OUP Academic, Oxford University Press, 10 Feb. 2022, academic.oup.com/book/41521/chapter-abstract/352955628?redirectedFrom=fulltext.
“HIPAA Violations & Enforcement.” American Medical Association, www.ama-assn.org/practice-management/hipaa/hipaa-violations-enforcement. Accessed 11 Sept. 2025.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients.
Contact MPI Today for Personal Assistance
MPI’s Drug Master File provides speed-to-market regulatory and technical support related to our packaging components for medical and pharmaceutical market clients